Hydration-dependent interaction of Na+, K+ with glycine: A comparative study of neutral and zwitterionic forms via DFT calculations
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
This study employs ab initio calculations to probe glycine-Na+/K+ interactions in water clusters. The results reveal that Na+-glycine complexes transition from neutral to zwitterionic configurations at six water molecules, while K+ systems achieve this shift at four. Proton transfer analysis shows that hydration can reduce zwitterion formation energy barriers. Multimethod analyses (IGMH, AIM) reveal that the binding strength of glycine-Na+/K+ initially decreases with hydration, with the weakest interactions occurring at the structural pre-transition stage from the neutral to the zwitterionic form. Energy decomposition analysis quantifies the dominan of electrostatic interactions. These results elucidate hydration-modulated amino acid-ion coordination dynamics critical for biological transport processes.
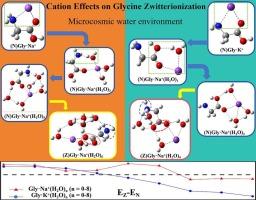
Na+, K+与甘氨酸的水合作用:通过DFT计算的中性和两性离子形式的比较研究
本研究采用从头计算来探测甘氨酸- na +/K+在水团簇中的相互作用。结果表明,Na+-甘氨酸配合物在6个水分子上从中性向两性离子构型转变,而K+体系在4个水分子上实现这种转变。质子转移分析表明,水合作用可以降低两性离子形成能垒。多方法分析(IGMH, AIM)表明,甘氨酸- na +/K+的结合强度最初随着水合作用而降低,最弱的相互作用发生在从中性形态到两性离子形态的结构过渡前阶段。能量分解分析量化了静电相互作用的优势。这些结果阐明了水合调节的氨基酸离子配位动力学对生物运输过程至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


