Immobilized nanodisks for study of ligand binding interactions
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The SpyCatcher/SpyTag system represents a unique technology that allows facile conjugation of proteins via formation of a covalent isopeptide bond between the 113 residue SpyCatcher protein and a 16 residue SpyTag peptide. Herein this technology was adapted to incorporate miniature bilayer membranes, termed nanodisks (ND). Fusion proteins comprised of apolipoprotein (apo) A-I/SpyTag peptide and SpyCatcher/maltose binding protein (MBP), respectively, were expressed and purified. Upon incubation of apoA-I:SpyTag fusion protein with SpyCatcher:MBP fusion protein, a covalent adduct was formed. ApoA-I:SpyTag formulated into ND particles with cardiolipin (CL) or phosphatidylcholine retained the ability to form an adduct with SpyCatcher:MBP. This adduct was then immobilized on amylose agarose resin beads through a binding interaction with the MBP component. Upon incubation of cytochrome c with immobilized CL ND, but not with phosphatidylcholine ND, cytochrome c binding occurred. When immobilized cytochrome c CL ND were incubated with buffer containing CaCl2, cytochrome c dissociated and was recovered in the supernatant fraction obtained after pelleting the amylose agarose beads. Subsequent incubation of the amylose agarose beads with 10 mM maltose revealed that nearly all of the cytochrome c had been released from the beads. The data are consistent with the known ability of calcium to form an ionic interaction with the two negatively charged phosphates in the polar head group of CL. Given the number of ligand-membrane interactions that occur in nature, immobilized ND provide a novel means to probe them.
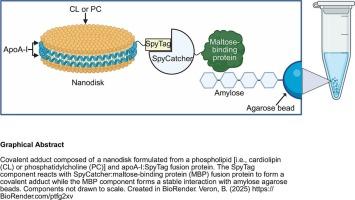
用于配体结合相互作用研究的固定化纳米圆盘
SpyCatcher/SpyTag系统代表了一种独特的技术,通过在113个残基SpyCatcher蛋白和16个残基SpyTag肽之间形成共价异肽键,可以轻松地将蛋白质偶联。在此,该技术被用于结合微型双层膜,称为纳米盘(ND)。分别表达并纯化了载脂蛋白A-I/SpyTag肽和SpyCatcher/麦芽糖结合蛋白(MBP)融合蛋白。将apoA-I:SpyTag融合蛋白与SpyCatcher:MBP融合蛋白孵育后,形成共价加合物。与心磷脂(CL)或磷脂酰胆碱配制成ND颗粒的ApoA-I:SpyTag保留了与SpyCatcher:MBP形成加合物的能力。然后通过与MBP组分的结合相互作用将该加合物固定在直链琼脂糖树脂珠上。细胞色素c与固定化CL ND孵育,但不与磷脂酰胆碱ND孵育,细胞色素c发生结合。将固定的细胞色素c CL ND与含有CaCl2的缓冲液孵育,细胞色素c解离,并在直链淀粉琼脂糖球成球后得到的上清部分中回收。随后将直链琼脂糖珠与10毫米麦芽糖孵育,发现几乎所有的细胞色素c都已从珠中释放出来。这些数据与已知的钙与CL极性头基团中两个带负电荷的磷酸盐形成离子相互作用的能力是一致的。鉴于自然界中发生的配体-膜相互作用的数量,固定化ND提供了一种新的方法来探测它们。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochimica et biophysica acta. Biomembranes
生物-生化与分子生物学
CiteScore
8.20
自引率
5.90%
发文量
175
审稿时长
2.3 months
期刊介绍:
BBA Biomembranes has its main focus on membrane structure, function and biomolecular organization, membrane proteins, receptors, channels and anchors, fluidity and composition, model membranes and liposomes, membrane surface studies and ligand interactions, transport studies, and membrane dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


