Transforming ureas into carbamates: From allosteric p38α MAPK ligands to dual BChE/p38α MAPK inhibitors
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Given the limited benefits of anticholinergic drugs and the repeated clinical failures of anti-amyloid therapies, the therapeutic focus in Alzheimer's disease (AD) is gradually shifting toward addressing both disease symptoms and its major underlying cause – neuroinflammation. We have developed novel multi-target directed ligands that inhibit butyrylcholinesterase (BChE) and p38α mitogen-activated protein kinase (p38α MAPK) to simultaneously target cholinergic deficits and neuroinflammation in AD. Following in silico design, we converted known allosteric pyrazolyl urea p38α MAPK ligands into N,N-disubstituted carbamates that pseudo-irreversibly inhibit hBChE while retaining p38α MAPK inhibitory activity. The lead compound 13a has favourable central nervous system (CNS) drug-like properties in vitro and shows procognitive effects in an in vivo scopolamine-induced amnesia model. Our series demonstrates that targeted structural modifications of selective kinase inhibitors, based on a comprehensive knowledge of cholinesterase structure and function, enable expansion of the effect to the CNS. This approach offers critical insights to pave the way for the development of novel dual-target agents that modulate both cholinergic and neuroinflammatory pathways in neurodegenerative diseases.
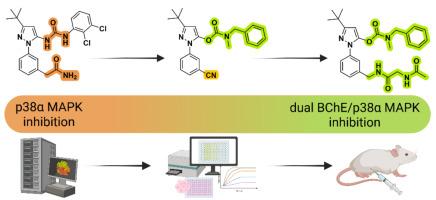
尿素转化为氨基甲酸酯:从变构p38α MAPK配体到双BChE/p38α MAPK抑制剂
鉴于抗胆碱能药物的有限疗效和抗淀粉样蛋白治疗的反复临床失败,阿尔茨海默病(AD)的治疗重点正逐渐转向解决疾病症状及其主要潜在原因-神经炎症。我们开发了一种新的多靶点定向配体,可以抑制丁基胆碱酯酶(BChE)和p38α丝裂原活化蛋白激酶(p38α MAPK),同时靶向阿尔茨海默病的胆碱能缺陷和神经炎症。根据硅设计,我们将已知的变构吡唑脲p38α MAPK配体转化为N,N-二取代氨基甲酸酯,其在保持p38α MAPK抑制活性的同时,对hBChE具有伪不可逆的抑制作用。先导化合物13a在体外具有良好的中枢神经系统(CNS)药物样特性,并在体内东莨菪碱诱导的健忘症模型中显示出促进认知的作用。我们的系列研究表明,基于对胆碱酯酶结构和功能的全面了解,选择性激酶抑制剂的靶向结构修饰可以将其作用扩展到中枢神经系统。这种方法提供了重要的见解,为开发调节神经退行性疾病中胆碱能和神经炎症途径的新型双靶点药物铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


