Photoinactivation of Leishmania amazonensis by Soranjidiol and white LED: Unraveling photodynamic and cell death mechanisms
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Cutaneous Leishmaniasis (CL) is part of the group of neglected diseases that pose significant global health challenges. Despite the many years since the discovery of pentavalent antimonials, the development of effective and affordable therapies remains a major obstacle. Antimicrobial photodynamic therapy (aPDT) is a localized treatment that has demonstrated efficacy in eliminating diverse pathogens without inducing resistance. Soranjidiol (Sor), a natural anthraquinone photosensitizer (PS), has shown promising in vitro and in vivo results against Leishmania amazonensis. This study aims to elucidate the photodynamic and cell death mechanisms of Sor by evaluating two different concentrations in the promastigote form and assessing its photoinactivation effects on the amastigote form of L. amazonensis.
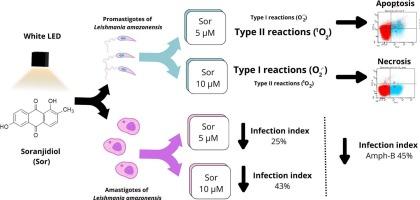
Our results demonstrate that Sor is a versatile and effective PS capable of eliminating promastigotes at low concentrations through the generation of reactive oxygen species and reactive nitrogen intermediates. Although aPDT is believed to generate both singlet oxygen and radicals, our findings indicate that type II reactions (1O₂) may be favoured at low Sor concentrations (singlet oxygen generation), leading to apoptosis, whereas type I reactions (superoxide anion) predominate at the highest concentration, resulting in necrosis.
The amastigote form was also successfully photo-inactivated, resulting in a reduction in the infection index comparable to that of Amphotericin B, a second-line drug for CL treatment. Although complete amastigote elimination was not achieved, the application of serial aPDT sessions could potentially improve therapeutic efficacy under optimized conditions and might represent a promising strategy to promote lesion healing.
Soranjidiol和白光LED对亚马逊利什曼原虫的光失活:揭示光动力学和细胞死亡机制
皮肤利什曼病是构成重大全球卫生挑战的被忽视疾病之一。尽管发现五价锑已有多年,但开发有效和负担得起的疗法仍然是一个主要障碍。抗菌素光动力疗法(aPDT)是一种局部治疗,已证明在消除多种病原体而不诱导耐药性的效果。Soranjidiol (Sor)是一种天然的蒽醌类光敏剂(PS),在体外和体内对亚马逊利什曼原虫(Leishmania amazonensis)均有良好的治疗效果。本研究旨在通过评价两种不同浓度的索尔在亚马逊L. promastigote形态中的光动力学和细胞死亡机制,并评估其对亚马逊L. amastigote形态的光失活作用。我们的研究结果表明,Sor是一种多功能和有效的PS,能够通过产生活性氧和活性氮中间体在低浓度下消除promastigotes。虽然aPDT被认为同时产生单线态氧和自由基,但我们的研究结果表明,在低Sor浓度(单线态氧生成)下,II型反应(o₂)可能更有利,导致细胞凋亡,而在最高浓度下,I型反应(超氧阴离子)占主导地位,导致坏死。无梭体形式也被成功地光灭活,导致感染指数的降低与两性霉素B(一种用于CL治疗的二线药物)相当。虽然没有完全消除无尾螺旋体,但在优化的条件下,连续aPDT的应用可能会提高治疗效果,并可能代表一种有希望的促进病变愈合的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


