Evaluation of the functional impact of rare CYP2C19 missense variants identified in understudied Populations: An Integrated in silico and in vitro analysis
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
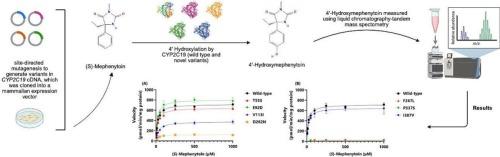
Cytochrome P450 enzymes, particularly CYP2D6 and CYP2C19, play a crucial role in metabolizing various prescribed medications. While common CYP2C19 variants, such as *2 and *3 alleles, are well-studied, rare and novel variants remain less understood, especially in understudied populations. This study investigated the functional impact of seven rare or novel CYP2C19 missense variants (p.T55S, p.E92D, p.V113I, p.D262N, p.F267L, p.P337S, and p.I387V) identified in the Emirati population, some of which have also been reported in other populations. In silico prediction programs and molecular modeling have been used to evaluate and predict the expected impacts of these variants. In addition, we employed site-directed mutagenesis to generate these variants in CYP2C19 cDNA, which was cloned into a mammalian expression vector, and evaluated their functional consequences using in vitro enzymatic assays. Our findings revealed that five of the seven variants (p.T55S, p.V113I, p.D262N, p.F267L, and p.P337S) significantly reduced CYP2C19 4′-hydroxylation catalytic activity towards (S)-mephenytoin, suggesting detrimental effects on drug metabolism. These results underscore the clinical importance of considering the impact of rare variants and, consequently, the need for their detailed functional analysis to integrate them into the implementation of pharmacogenomics and personalized medicine. This research contributes to the growing understanding of population-specific genetic variations in CYP2C19 and their potential implications for the response and safety of a significant number of medications metabolized by this enzyme.
在未充分研究的人群中鉴定的罕见CYP2C19错义变异对功能影响的评估:集成的计算机和体外分析
细胞色素P450酶,特别是CYP2D6和CYP2C19,在各种处方药的代谢中起着至关重要的作用。虽然常见的CYP2C19变异,如*2和*3等位基因,已经得到了很好的研究,但罕见和新颖的变异仍然知之甚少,特别是在研究不足的人群中。本研究调查了在阿联酋人群中发现的七种罕见或新型CYP2C19错义变异(p.T55S, p.E92D, p.V113I, p.D262N, p.F267L, p.P337S和p.I387V)的功能影响,其中一些在其他人群中也有报道。计算机预测程序和分子模型已被用于评估和预测这些变异的预期影响。此外,我们采用位点定向诱变技术在CYP2C19 cDNA中产生这些变异,并将其克隆到哺乳动物表达载体中,并使用体外酶分析评估其功能后果。我们的研究结果显示,七个变体中的五个(p.T55S, p.V113I, p.D262N, p.F267L和p.P337S)显著降低了CYP2C19 4 ' -羟化对(S)-甲苯托英的催化活性,表明对药物代谢有不利影响。这些结果强调了考虑罕见变异影响的临床重要性,因此,需要对其进行详细的功能分析,以将其整合到药物基因组学和个性化医疗的实施中。这项研究有助于加深对CYP2C19人群特异性遗传变异的理解,以及它们对大量由该酶代谢的药物的反应和安全性的潜在影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


