Benzyl Thioether: A Dynamic Covalent Motif for Covalent Adaptable Networks
IF 5.2
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
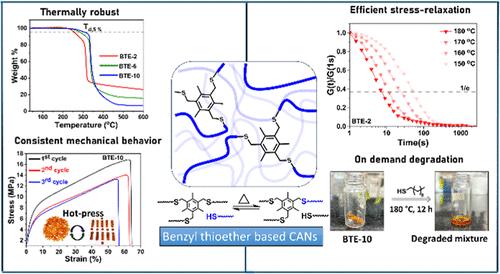
Covalent adaptable networks (CANs) have emerged as a promising alternative to conventional thermoset polymers, offering reprocessability and recyclability through the incorporation of dynamic covalent linkages. However, dynamic linkages often compromise the thermal and chemical stability of the polymer, which is essential for many thermosets’ applications. Designing a chemistry that imparts both robustness and efficient dynamicity to the cross-linked polymers is a major challenge. To this end, this report introduces benzyl thioether as a robust and dynamic covalent motif for designing CANs. First, using the small molecular model studies, the dynamic exchange in the thioether was demonstrated and a linear polythioether bearing benzyl thioether was prepared. The CANs were prepared by solvent-free melt polymerization of trifunctional benzyl ether with a linear dithiol. Further on, a series of CANs was prepared, and the properties of these CANs, which include swelling, glass-transition temperature (Tg), and mechanical properties, were tailored by regulating the dithiol chain lengths. The CANs demonstrated excellent thermal and chemical stability owing to the presence of robust thioether linkages. The reprocessability and efficient relaxation of the imposed stress in the CANs at elevated temperature were made possible by the dynamic exchange capability of the benzyl thioether linkage. Impressively, the dynamic behaviors of the CANs were shown to be regulated by monomer chain lengths as well as the catalyst concentration. Finally, degradation of the CANs in the presence of excess thiol was demonstrated. To the best of our knowledge, this is the first study that introduces the benzyl thioether motif for designing the robust and dynamic transthioetherification-based CAN, allowing for the fine-tuning of material properties. This approach will pave the way for the design of robust and sustainable polymeric materials.
苄基硫醚:共价自适应网络的动态共价基序
共价自适应网络(can)已成为传统热固性聚合物的一种有前途的替代品,通过结合动态共价键提供可再加工性和可回收性。然而,动态连接通常会损害聚合物的热稳定性和化学稳定性,这对于许多热固性材料的应用至关重要。设计一种既能赋予交联聚合物坚固性又能赋予其高效动力学的化学物质是一项重大挑战。为此,本报告介绍了苄基硫醚作为设计can的稳健和动态共价基序。首先,利用小分子模型研究了硫醚中的动态交换,制备了含苄基硫醚的线性聚硫醚。采用三官能团苯醚与线性二硫醇的无溶剂熔融聚合法制备了can。在此基础上,制备了一系列can,并通过调节二硫醇链的长度来调整can的溶胀、玻璃化转变温度(Tg)和力学性能。由于存在强大的硫醚键,can表现出优异的热稳定性和化学稳定性。苄基硫醚键的动态交换能力使can在高温下的再加工性和有效的应力松弛成为可能。令人印象深刻的是,can的动力学行为受到单体链长度和催化剂浓度的调节。最后,证明了在过量硫醇存在下can的降解。据我们所知,这是第一个引入苄基硫醚基序来设计基于转硫醚化的稳健和动态CAN的研究,允许对材料性能进行微调。这种方法将为设计坚固耐用的聚合物材料铺平道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


