Simultaneous Construction of Planar and C–N Axial Chirality Ferrocenes by Pd-Catalyzed Cascade Reaction: Reciprocal Carbonyl-Carbonyl Interaction
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A Pd-catalyzed cascade combining asymmetric intramolecular C–H arylation and intermolecular Buchwald-Hartwig coupling constructs ferrocenes with both planar and C–N axial chirality (75% yield, 99% ee, 15:1 d.r.). Kinetic experiments and in situ 31P NMR confirmed the sequence of the cascade reactions. Moreover, this chirality stems from n−π* interactions and sterics. These ferrocenes are converted diastereoselectively (>20:1 dr) into planar/central chiral phosphine ligands. These ligands enable Pd-catalyzed enantioselective allylic alkylation (85% yield, 97% ee).
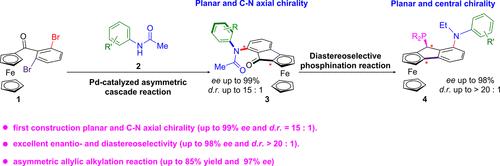
用pd催化级联反应同时构建平面和C-N轴向手性二茂铁:羰基-羰基相互作用。
一个pd催化的级联反应结合了不对称分子内C-H基化和分子间Buchwald-Hartwig偶联,构建了具有平面和C-N轴向手性的二茂铁(产率75%,ee 99%, dr值15:1)。动力学实验和原位核磁共振证实了级联反应的顺序。此外,这种手性源于n-π*相互作用和立体构型。这些二茂铁被非对映选择性地(>20:1 dr)转化为平面/中心手性膦配体。这些配体使pd催化的烯丙基对映选择性烷基化(产率85%,ee 97%)成为可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


