Slc7a7 licenses macrophage glutaminolysis for restorative functions in atherosclerosis
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Atherosclerosis is a life-threatening condition characterized by chronic inflammation of the arterial wall. Atherosclerotic plaque macrophages are key players at the site of disease, where metabolic reprogramming dictates the progression of pathogenesis. Here we show that reduced macrophage glutaminase activity is related to glutaminase (GLS)-1 and not GLS2 expression. While glutamine synthetase serves as a metabolic rheostat controlling nutrient flux into cells in vitro, macrophage restorative functions in the context of atherosclerosis relies more heavily on glutamine influx. Enhanced glutamine flux is largely mediated by the SLC7A7 exchanger in macrophages: Slc7a7-silenced macrophages have reduced glutamine influx and GLS1-dependent glutaminolysis, impeding downstream signalling involved in macrophage restorative functions. In vivo, macrophage-specific deletion of Slc7a7 accelerates atherosclerosis in mice with more complex necrotic core composition. Finally, cell-intrinsic regulation of glutaminolysis drives macrophage metabolic and transcriptional rewiring in atherosclerosis by diverting exogenous Gln flux to balance remodelling and restorative functions. Thus, we uncover a role of SLC7A7-dependent glutamine uptake upstream of glutaminolysis in atherosclerotic plaque development and stability. The authors provide a comprehensive characterization of how glutamine uptake and utilization regulate macrophage function in atherosclerosis.
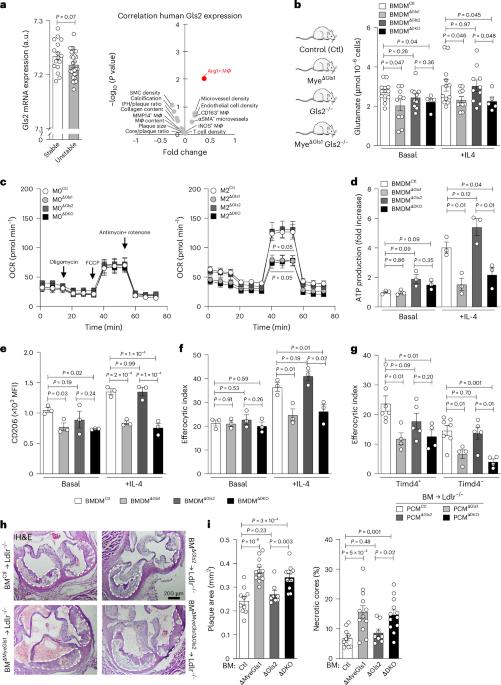
Slc7a7允许巨噬细胞谷氨酰胺溶解在动脉粥样硬化中的恢复功能。
动脉粥样硬化是一种危及生命的疾病,其特征是动脉壁的慢性炎症。动脉粥样硬化斑块巨噬细胞是疾病发生部位的关键参与者,代谢重编程决定了发病机制的进展。本研究表明,巨噬细胞谷氨酰胺酶活性降低与谷氨酰胺酶(GLS)-1表达有关,而与GLS2表达无关。虽然谷氨酰胺合成酶在体外作为代谢变换器控制营养物质进入细胞,但动脉粥样硬化背景下巨噬细胞的恢复功能更多地依赖于谷氨酰胺内流。巨噬细胞中SLC7A7交换器介导谷氨酰胺通量增强:SLC7A7沉默的巨噬细胞减少谷氨酰胺内流和gls1依赖的谷氨酰胺水解,阻碍参与巨噬细胞恢复功能的下游信号传导。在体内,巨噬细胞特异性缺失Slc7a7会加速具有更复杂坏死核心成分的小鼠的动脉粥样硬化。最后,通过转移外源性谷氨酰胺通量来平衡重塑和恢复功能,细胞内的谷氨酰胺溶解调节驱动动脉粥样硬化中巨噬细胞的代谢和转录重新连接。因此,我们揭示了slc7a7依赖性谷氨酰胺摄取上游谷氨酰胺溶解在动脉粥样硬化斑块发展和稳定中的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


