Tanshinone IIA suppresses endoplasmic reticulum stress-mediated IRE1α/TXNIP/NLRP3 pathway to mitigate pyroptosis in type 2 diabetic osteoporosis
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Type 2 diabetic osteoporosis (T2DOP) is a chronic skeletal disorder affecting bone structure and strength. Research has shown that endoplasmic reticulum stress (ERS) activates the NLRP3 inflammasome and osteoblast pyroptosis through inositol-requiring enzyme 1 alpha (IRE1α) and its interaction with thioredoxin-interacting protein (TXNIP). Tanshinone IIA (Tan IIA), a naturally occurring lipophilic diterpene derived from Salvia miltiorrhiza, inhibits the activation of IRE1α. This study explored Tan IIA’s therapeutic effects on T2DOP. MC3T3-E1 cells were cultured in a high-glucose medium to replicate conditions characteristic of the diabetic environment. Additionally, a T2DOP mouse model was developed through the intraperitoneal injection of streptozotocin in conjunction with a 60 %kacl high fat diet. Tan IIA was administered at different concentrations. Cell viability, differentiation and mineralization was assessed via CCK-8 assays, alkaline phosphatase staining, and Alizarin Red S staining. Serum biochemical markers were quantified using ELISA kits, while bone quality was evaluated using micro-CT and histological analysis. The mechanism was confirmed by Western blot, immunohistochemistry, RT-qPCR, molecular docking, and Cellular Thermal Shift Assay. Results showed Tan IIA improved insulin resistance, reduced inflammation and oxidative stress, and restored bone mass in T2DOP mice. Mechanistically, Tan IIA inhibited the IRE1α/TXNIP/NLRP3 pathway in vivo and in vitro, and ERS inducer tunicamycin attenuated its beneficial effects. The data establish Tan IIA as a promising candidate for treating T2DOP by alleviating osteoblast dysfunction and bone loss via the ERS-mediated IRE1α/TXNIP/NLRP3 pathway and pyroptosis.
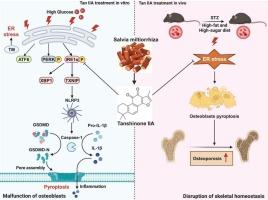
丹参酮IIA抑制内质网应激介导的IRE1α/TXNIP/NLRP3通路减轻2型糖尿病骨质疏松症的焦下垂。
2型糖尿病骨质疏松症(T2DOP)是一种影响骨骼结构和强度的慢性骨骼疾病。研究表明,内质网应激(ERS)通过肌醇要求酶1α (IRE1α)及其与硫氧还蛋白相互作用(TXNIP)的相互作用激活NLRP3炎性体和成骨细胞热亡。丹参酮IIA (Tan IIA)是一种天然存在的亲脂二萜,从丹参中提取,可抑制IRE1α的激活。本研究探讨坦IIA对T2DOP的治疗作用。MC3T3-E1细胞在高糖培养基中培养,以复制糖尿病环境的特征。此外,通过腹腔注射链脲佐菌素结合60 %kacl高脂肪饮食,建立T2DOP小鼠模型。注射不同浓度的Tan IIA。通过CCK-8染色、碱性磷酸酶染色和茜素红S染色评估细胞活力、分化和矿化。采用酶联免疫吸附试验(ELISA)定量血清生化指标,显微ct及组织学分析评价骨质量。Western blot、免疫组织化学、RT-qPCR、分子对接、细胞热移实验证实了其作用机制。结果显示,Tan IIA改善T2DOP小鼠胰岛素抵抗,降低炎症和氧化应激,恢复骨量。在机制上,Tan IIA在体内和体外抑制IRE1α/TXNIP/NLRP3通路,ERS诱导剂tunicamycin减弱其有益作用。这些数据表明,Tan IIA通过ers介导的IRE1α/TXNIP/NLRP3通路和焦噬减轻成骨细胞功能障碍和骨质流失,是治疗T2DOP的有希望的候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


