Decoding the nitration-phosphorylation switch: 7,8-Dihydroxyflavone rebalances TrkB signaling dynamics for retinal neuroprotection
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Purpose
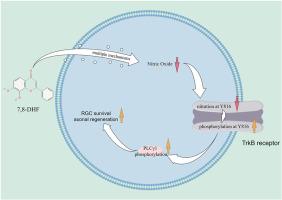
To investigate how 7,8-Dihydroxyflavone (7,8-DHF) coordinates competing post-translational modifications of TrkB, specifically examining its role in modulating the redox-sensitive balance between tyrosine nitration and phosphorylation to achieve neuroprotective efficacy.
Methods
The effects of 7,8-DHF on nitric oxide (NO) inhibition, protein nitration, TrkB activation, and downstream signaling were assessed using a male mouse optic nerve crush (ONC) model and 3D retinal explants. Techniques such as Western blotting, ELISA, immunoprecipitation, immunohistochemistry, metabolomics, and molecular docking were used to examine the biochemical and molecular impacts of 7,8-DHF.
Results
7,8-DHF reduced NO levels and inducible/neuronal nitric oxide synthase (iNOS/nNOS) expression in 3D retinal cultures and ONC mouse models, with molecular docking indicating its binding to iNOS's active site and metabolomics showing downregulation of the arginine biosynthesis pathway. It also decreased TrkB nitration in vivo and in vitro, with the Y816 residue being critical for this modification. 7,8-DHF enhanced TrkB phosphorylation at Y816 and PLCγ1 activation, inhibited by L-arginine and TrkB blockers. Additionally, 7,8-DHF promoted retinal gangion cell survival and axonal regeneration via TrkB signaling, effects reversed by nitration agents or TrkB blockers.
Conclusion
7,8-DHF pioneers a new class of nitration-phosphorylation modulators, converting nitrosative damage into neuroregenerative signals by rebalancing TrkB's modification states. This mechanistic insight opens therapeutic avenues for optic nerve disorders.
解码硝化-磷酸化开关:7,8-二羟黄酮重新平衡TrkB信号动力学对视网膜神经保护
目的研究7,8-二羟黄酮(7,8- dhf)如何协调TrkB的竞争性翻译后修饰,特别是研究其在调节酪氨酸硝化和磷酸化之间的氧化还原敏感平衡以实现神经保护作用中的作用。方法采用雄性小鼠视神经挤压(ONC)模型和3D视网膜外植体,观察7,8- dhf对一氧化氮(NO)抑制、蛋白硝化、TrkB激活和下游信号传导的影响。采用Western blotting、ELISA、免疫沉淀、免疫组织化学、代谢组学和分子对接等技术检测7,8- dhf的生化和分子影响。结果7,8- dhf在3D视网膜培养和ONC小鼠模型中降低NO水平和诱导型/神经元型一氧化氮合酶(iNOS/nNOS)表达,分子对接表明其与iNOS活性位点结合,代谢组学显示精氨酸生物合成途径下调。它还降低了体内和体外TrkB的硝化作用,其中Y816残基对这种修饰至关重要。7,8- dhf增强了TrkB在Y816位点的磷酸化和plc γ - 1的激活,被l -精氨酸和TrkB阻滞剂抑制。此外,7,8- dhf通过TrkB信号传导促进视网膜神经节细胞存活和轴突再生,这一作用被硝化剂或TrkB阻滞剂逆转。结论7,8- dhf是一类新的硝化磷酸化调节剂的先驱,通过重新平衡TrkB的修饰状态,将亚硝化损伤转化为神经再生信号。这种机制的见解为视神经疾病开辟了治疗途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


