Green decorated silver nanoparticles over the surface of graphene oxide: Investigation of its catalytic performance for synthesis of 5-substituted 1H-tetrazoles
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
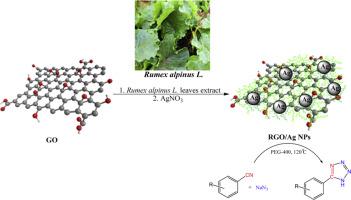
Herein an innovative and environmentally friendly method for supporting silver nanoparticles (Ag NPs) on graphene oxide that has been modified by Rumex alpinus L. leaf extract was represented. The RGO/Ag NPs was designed as a result of the phytoconstituents in the extract of Rumex alpinus L. leaves and the biogenic reduction of silver ions on graphene oxide, which was made possible by the presence of polyhydroxyl groups. ICP, XRD, TEM, elemental mapping, FE-SEM, and EDX were used to characterize the generated nanocomposite. As demonstrated by TEM imaging, the silver nanoparticles were mono-dispersed, spherical, and roughly 20–30 nm in diameter. The catalytic performance of the RGO/Ag NPs was investigated for the making 5-substituted 1H-tetrazoles from nitriles and sodium azide. A range of different aryl nitriles were applied to make tetrazoles with good yields via [3 + 2] cycloaddition. The RGO/Ag NPs catalyst shown good recyclability until 7 runs without significant decrease in efficiency.
氧化石墨烯表面的绿色修饰银纳米粒子:其合成5-取代1h -四唑的催化性能研究
本文提出了一种创新和环保的方法,在氧化石墨烯上负载银纳米粒子(Ag NPs),氧化石墨烯是由芦梅叶提取物修饰的。RGO/Ag NPs的设计是基于芦笋叶提取物中的植物成分和氧化石墨烯上银离子的生物还原,这是由于多羟基的存在而实现的。采用ICP、XRD、TEM、元素映射、FE-SEM和EDX对合成的纳米复合材料进行表征。TEM成像显示,银纳米颗粒呈单分散球形,直径约为20-30 nm。研究了还原氧化石墨烯/银纳米粒子对腈和叠氮化钠合成5-取代1h -四唑的催化性能。采用[3 + 2]环加成法制备了一系列不同的芳基腈,收率较高。RGO/Ag NPs催化剂在7次循环前表现出良好的可回收性,且效率没有明显下降。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


