Horse heart mini- and full length-myoglobin: pH effects on CO binding
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
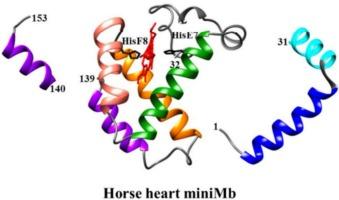
Mini-myoglobin (mini-HH-Mb) is a proteolytic fragment of horse heart myoglobin (HH-Mb) comprising residues 32–139, grossly corresponding to the central exon of the HH-Mb gene, which encodes residues 31–105. Unlike HH-Mb, which displays a single exponential for both CO association and CO dissociation kinetics, mini-HH-Mb shows a biphasic kinetic behavior for both processes, indicating the presence of at least two distinct conformations which are in a very slow (or no) equilibrium with each other. Between pH 2 and 12, CO association to both species of mini-HH-Mb shows two proton-linked transitions, one in the neutral-alkaline pH range (not observed for HH-Mb) and a second one in the acidic region displaying a pKa of 2.9 like that observed in HH-Mb (pKa = 2.7). Kinetics of CO dissociation from both species of mini-HH-Mb-CO was investigated between pH 5.5 and 10.5 only, since outside this pH range the slow CO dissociation kinetics are affected by protein denaturation, which shows up after few seconds. The CO dissociation rate shows a bell-shaped pH dependence for both conformations, while ligand dissociation from HH-Mb-CO is pH-independent. These features find a structural basis on molecular modelling, displaying a higher flexibility of both the proximal and distal side of the heme pocket in mini-HH-Mb, envisaging multiple conformations with different reactivity. This indicates that mini-HH-Mb differs from HH-Mb, suggesting a significant structural-functional role for the N- and C-terminal regions in O2 supply to highly demanding tissues, like the retina, with implications for improving retinal blood flow in ocular pathologies.
马心脏微型和全长肌红蛋白:pH值对CO结合的影响
迷你肌红蛋白(mini-HH-Mb)是马心脏肌红蛋白(HH-Mb)的蛋白水解片段,包含残基32-139,大致对应于HH-Mb基因的中心外显子,编码残基31-105。与HH-Mb不同的是,mini-HH-Mb在CO结合和CO解离动力学上都表现出单一指数,而mini-HH-Mb在这两个过程中都表现出双相动力学行为,这表明至少存在两种不同的构象,它们彼此之间处于非常缓慢(或没有)的平衡状态。在pH值2 ~ 12之间,CO与两种mini-HH-Mb的结合均表现出两个质子连接的转变,一个在pH值为中性碱性的范围内(HH-Mb未观察到),另一个在pH值为酸性的区域,其pKa为2.9,与HH-Mb相似(pKa = 2.7)。两种mini-HH-Mb-CO的CO解离动力学仅在pH 5.5和10.5之间进行了研究,因为在该pH范围之外,CO的缓慢解离动力学受到蛋白质变性的影响,这种变性在几秒钟后出现。两种构象的CO解离速率均与pH呈钟形关系,而HH-Mb-CO的配体解离速率与pH无关。这些特征在分子模型上找到了结构基础,在mini-HH-Mb中血红素口袋的近端和远端都显示出更高的灵活性,设想了具有不同反应性的多种构象。这表明mini-HH-Mb与HH-Mb不同,提示N端和c端区域在向高需求组织(如视网膜)供应氧气方面具有重要的结构功能作用,这可能有助于改善眼部病变中的视网膜血流量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


