ADNP-15: An Open-Source Histopathological Dataset for Neuritic Plaque Segmentation in Human Brain Whole Slide Images with Frequency Domain Image Enhancement for Stain Normalization
IF 4.2
4区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
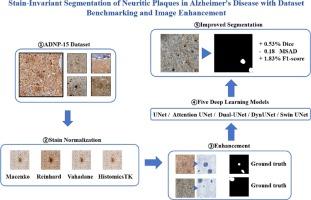
Alzheimer's Disease (AD) is a neurodegenerative disorder characterized by amyloid-β plaques and tau neurofibrillary tangles, which serve as key histopathological features. The identification and segmentation of these lesions are crucial for understanding AD progression but remain challenging due to the lack of large-scale annotated datasets and the impact of staining variations on automated image analysis. Deep learning has emerged as a powerful tool for pathology image segmentation; however, model performance is significantly influenced by variations in staining characteristics, necessitating effective stain normalization and enhancement techniques. In this study, we address these challenges by introducing an open-source dataset (ADNP-15) of neuritic plaques (i.e., amyloid deposits combined with a crown of dystrophic tau-positive neurites) in human brain whole slide images. We establish a comprehensive benchmark by evaluating five widely adopted deep learning models across four stain normalization techniques, providing deeper insights into their influence on neuritic plaque segmentation. Additionally, we propose a novel image enhancement method that improves segmentation accuracy, particularly in complex tissue structures, by enhancing structural details and mitigating staining inconsistencies. Our experimental results demonstrate that this enhancement strategy significantly boosts model generalization and segmentation accuracy. All datasets and code are open-source, ensuring transparency and reproducibility while enabling further advancements in the field.
ADNP-15:一个开源的组织病理学数据集,用于人脑全切片图像的神经斑块分割,并使用频域图像增强进行染色归一化
阿尔茨海默病(AD)是一种神经退行性疾病,其特征是淀粉样蛋白-β斑块和tau神经原纤维缠结,这是关键的组织病理学特征。这些病变的识别和分割对于了解AD的进展至关重要,但由于缺乏大规模的注释数据集和染色变化对自动图像分析的影响,仍然具有挑战性。深度学习已经成为病理图像分割的有力工具;然而,模型性能受到染色特征变化的显著影响,需要有效的染色归一化和增强技术。在这项研究中,我们通过引入人类大脑全幻灯片图像中的神经斑块(即淀粉样蛋白沉积与营养不良的tau阳性神经突冠相结合)的开源数据集(ADNP-15)来解决这些挑战。我们通过评估四种染色归一化技术中五种广泛采用的深度学习模型,建立了全面的基准,从而更深入地了解它们对神经斑块分割的影响。此外,我们提出了一种新的图像增强方法,通过增强结构细节和减轻染色不一致来提高分割精度,特别是在复杂的组织结构中。实验结果表明,该增强策略显著提高了模型泛化和分割精度。所有数据集和代码都是开源的,确保了透明度和可再现性,同时促进了该领域的进一步发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Irbm
ENGINEERING, BIOMEDICAL-
CiteScore
10.30
自引率
4.20%
发文量
81
审稿时长
57 days
期刊介绍:
IRBM is the journal of the AGBM (Alliance for engineering in Biology an Medicine / Alliance pour le génie biologique et médical) and the SFGBM (BioMedical Engineering French Society / Société française de génie biologique médical) and the AFIB (French Association of Biomedical Engineers / Association française des ingénieurs biomédicaux).
As a vehicle of information and knowledge in the field of biomedical technologies, IRBM is devoted to fundamental as well as clinical research. Biomedical engineering and use of new technologies are the cornerstones of IRBM, providing authors and users with the latest information. Its six issues per year propose reviews (state-of-the-art and current knowledge), original articles directed at fundamental research and articles focusing on biomedical engineering. All articles are submitted to peer reviewers acting as guarantors for IRBM''s scientific and medical content. The field covered by IRBM includes all the discipline of Biomedical engineering. Thereby, the type of papers published include those that cover the technological and methodological development in:
-Physiological and Biological Signal processing (EEG, MEG, ECG…)-
Medical Image processing-
Biomechanics-
Biomaterials-
Medical Physics-
Biophysics-
Physiological and Biological Sensors-
Information technologies in healthcare-
Disability research-
Computational physiology-
…
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


