Nanomedicine strategies for cuproptosis: Metabolic reprogramming and tumor immunotherapy
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Cuproptosis, a recently discovered form of regulated cell death involving copper ion metabolism, has emerged as a promising approach for tumor therapy. This pathway not only directly eliminates tumor cells but also promotes immunogenic cell death (ICD), reshaping the tumor microenvironment (TME) and initiating robust anti-tumor immune responses. However, translating cuproptosis-based therapies into clinical applications is hindered by challenges, including complex metabolic regulation, TME heterogeneity, and the precision required for effective drug delivery. To address these limitations, nanoparticles offer transformative solutions by providing precise delivery of cuproptosis-inducing agents, controlled drug release, and enhanced therapeutic efficacy through simultaneous modulation of metabolic pathways and immune responses. This review systematically discusses recent advancements in nanoparticle-based cuproptosis delivery systems, highlighting nanoparticle design principles and their synergistic effects when integrated with other therapeutic modalities such as ICB, PTT, and CDT. Furthermore, we explore the potential of cuproptosis-based nanomedicine for personalized cancer treatment by emphasizing strategies for TME stratification and therapeutic optimization tailored to patient profiles. By integrating current insights from metabolic reprogramming, tumor immunotherapy, and nanotechnology, this review aims to facilitate the clinical translation of cuproptosis nanomedicine and significantly contribute to the advancement of precision oncology.
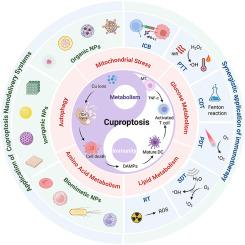
纳米药物治疗铜骨畸形:代谢重编程和肿瘤免疫治疗
铜坏死是最近发现的一种涉及铜离子代谢的受调节细胞死亡形式,已成为一种有希望的肿瘤治疗方法。该途径不仅直接消除肿瘤细胞,而且促进免疫原性细胞死亡(ICD),重塑肿瘤微环境(TME)并启动强大的抗肿瘤免疫应答。然而,将基于铜裂的治疗方法转化为临床应用受到各种挑战的阻碍,包括复杂的代谢调节、TME异质性和有效给药所需的精度。为了解决这些限制,纳米颗粒提供了变革性的解决方案,通过提供铜中毒诱导剂的精确递送,控制药物释放,并通过同时调节代谢途径和免疫反应提高治疗效果。这篇综述系统地讨论了基于纳米颗粒的铜沉淀输送系统的最新进展,强调了纳米颗粒的设计原理及其与其他治疗方式(如ICB、PTT和CDT)结合时的协同效应。此外,我们通过强调TME分层策略和根据患者情况量身定制的治疗优化,探索了基于铜体病的纳米医学在个性化癌症治疗中的潜力。通过整合代谢重编程、肿瘤免疫治疗和纳米技术的最新见解,本综述旨在促进铜突病纳米医学的临床转化,并为精确肿瘤学的发展做出重大贡献。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


