Yeast-two-hybrid based high-throughput screening to discover SARS-CoV-2 fusion inhibitors by targeting the HR1/HR2 interaction
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The continuous emergence of SARS-CoV-2 variants as well as other potential future coronavirus has challenged the effectiveness of current COVID-19 vaccines. Therefore, there remains a need for alternative antivirals that target processes less susceptible to mutations, such as the formation of six-helix bundle (6-HB) during the viral fusion step of host cell entry. In this study, a novel high-throughput screening (HTS) assay employing a yeast-two-hybrid (Y2H) system was established to identify inhibitors of HR1/HR2 interaction. The compound IMB-9C, which achieved single-digit micromolar inhibition of SARS-CoV-2 and its Omicron variants with low cytotoxicity, was selected. IMB-9C effectively blocks the HR1/HR2 interaction in vitro and inhibits SARS-CoV-2-S-mediated cell–cell fusion. It binds to both HR1 and HR2 through non-covalent interaction and influences the secondary structure of HR1/HR2 complex. In addition, virtual docking and site-mutagenesis results suggest that amino acid residues A930, I931, K933, T941, and L945 are critical for IMB-9C binding to HR1. Collectively, in this study, we have developed a novel screening method for HR1/HR2 interaction inhibitors and identified IMB-9C as a potential antiviral small molecule against COVID-19 and its variants.
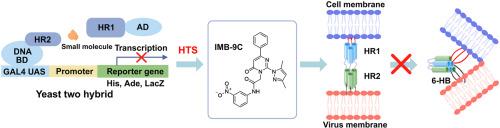
基于酵母-双杂交的高通量筛选发现针对HR1/HR2相互作用的SARS-CoV-2融合抑制剂
SARS-CoV-2变体以及其他潜在的未来冠状病毒的不断出现,对当前COVID-19疫苗的有效性提出了挑战。因此,仍然需要替代抗病毒药物,以靶向不易受突变影响的过程,例如在病毒进入宿主细胞的融合步骤中形成六螺旋束(6-HB)。在这项研究中,利用酵母-双杂交(Y2H)系统建立了一种新的高通量筛选(HTS)方法来鉴定HR1/HR2相互作用的抑制剂。选择了对SARS-CoV-2及其Omicron变体具有低细胞毒性的个位数微摩尔抑制的化合物IMB-9C。IMB-9C在体外有效阻断HR1/HR2相互作用,抑制sars - cov -2- s介导的细胞-细胞融合。它通过非共价相互作用与HR1和HR2结合,并影响HR1/HR2复合物的二级结构。此外,虚拟对接和位点诱变结果表明,氨基酸残基A930、I931、K933、T941和L945是IMB-9C与HR1结合的关键。总之,在本研究中,我们开发了一种新的HR1/HR2相互作用抑制剂筛选方法,并鉴定出IMB-9C是一种潜在的抗病毒小分子,可用于对抗COVID-19及其变体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


