Astrocyte FGF7/FGFR2 autocrine signaling mediates neuroinflammation and promotes MPTP-induced degeneration of dopaminergic neurons
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Reactive astrocytes, which exhibit a correlation with the degeneration of dopaminergic neurons, are present in a considerable number during the progression of Parkinson's disease (PD). However, the underlying factors shaping astrocyte reactivity and neuroinflammation in PD remain inadequately elucidated. Here, we demonstrate that fibroblast growth factor 7 (FGF7)/FGF receptor 2 (FGFR2) autocrine signaling intensifies astrocyte reactivity and inflammation. Genetic deletion of Arrb2, β-Arrestin2 encoding gene, led to escalated astrocyte reactivity in MPTP-treated mice, which was further substantiated in astrocyte-specific Arrb2 knockdown mice. RNA sequencing profiling of Arrb2 knockout astrocytes identified Fgf7 as a critical effector of astrocyte reactivity. Subsequently, conditional knockdown of Fgf7 and its receptor Fgfr2 in astrocytes elicited advantageous effects for MPTP-treated mice by restraining the inflammatory phenotypic transition of reactive astrocytes. Furthermore, deletion of astrocytic Fgf7 mitigated MPTP-induced pathology in Arrb2 knockout mice. Mechanistically, STAT1 was distinguished as the transcription factor suppressing Fgf7 expression, while β-Arrestin2 counteracted the proteasomal degradation of STAT1 by binding to RNF220, an E3 ubiquitin ligase for STAT1. More importantly, selectively engaging dopamine D2 receptor (Drd2)/β-Arrestin2-biased signaling using the agonist UNC9995 exhibited therapeutic potential in MPTP-treated mice via moderation of astrocytic FGF7 production, thereby restoring balance in astrocyte reactivity. Collectively, our study bridges a crucial knowledge gap by elucidating the novel functions of FGF family members within the central nervous system, particularly within the context of PD. The autocrine signaling of FGF7/FGFR2 represents a novel mechanism and a potential druggable target for modulating astrocyte-derived inflammation.
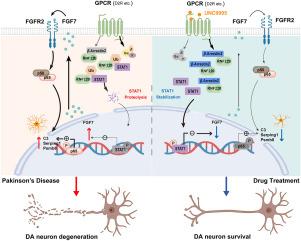
星形胶质细胞FGF7/FGFR2自分泌信号介导神经炎症并促进mptp诱导的多巴胺能神经元变性
与多巴胺能神经元退化相关的反应性星形胶质细胞在帕金森病(PD)的进展过程中大量存在。然而,形成星形胶质细胞反应性和PD神经炎症的潜在因素仍未充分阐明。在这里,我们证明了成纤维细胞生长因子7 (FGF7)/FGF受体2 (FGFR2)自分泌信号增强星形胶质细胞的反应性和炎症。β-Arrestin2编码基因Arrb2的基因缺失导致mptp处理小鼠星形胶质细胞反应性升高,这在星形胶质细胞特异性Arrb2敲低小鼠中得到进一步证实。rarrb2敲除星形胶质细胞的RNA测序分析发现Fgf7是星形胶质细胞反应性的关键效应因子。随后,星形胶质细胞中Fgf7及其受体Fgfr2的条件敲低,通过抑制反应性星形胶质细胞的炎症表型转变,对mptp处理的小鼠产生有利影响。此外,星形细胞Fgf7的缺失减轻了mptp诱导的Arrb2敲除小鼠的病理。从机制上讲,STAT1被认为是抑制Fgf7表达的转录因子,而β-Arrestin2通过结合STAT1的E3泛素连接酶RNF220来抵消STAT1的蛋白酶体降解。更重要的是,使用激动剂UNC9995选择性地参与多巴胺D2受体(Drd2)/β- arrestin2偏向信号传导,通过调节星形胶质细胞FGF7的产生,在mptp治疗的小鼠中显示出治疗潜力,从而恢复星形胶质细胞反应性的平衡。总的来说,我们的研究通过阐明FGF家族成员在中枢神经系统中的新功能,特别是在PD的背景下,弥合了一个关键的知识差距。FGF7/FGFR2的自分泌信号代表了一种调节星形胶质细胞来源的炎症的新机制和潜在的药物靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


