Room-temperature volatile organosulfurs for synthesis of hierarchical Cu7S4 hollow nanotubelets for photodegradation of organic pollutants
IF 3.3
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Herein, a novel, eco-friendly and sustainable green approach has developed for synthesis of uniform hollow Cu7S4 nanotubes on Cu foam using volatile organosulfur compounds of Allium Sativum L for photocatalytic of organic pollutants. This novel method facilitates sulfurization of Cu-foam without the need for surfactants (i.e regent free) at room temperature, resulting in Cu7S4 with unique hollow nanotubelets surface. The scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS) were employed to analysis the morphology, composition, structural phase and chemical states of the obtained sample. The N2 adsorption-desorption isotherm depicts the Brunauer-Emmett–Teller (BET) surface area of Cu7S4 hollow nanotubelets is about 7.2 m2 g−1 with a pore size distribution of 46.89 nm. The obtained Cu7S4@Cu-foam was applied for degradation of methylene blue (MB) and methyl orange (MO) pollutants under visible light irradiation and exhibited an enhanced photocatalytic activity. The pseudo first order degradation kinetics rates for MB and MO are found to be 0.012 and 0.0098 min−1, respectively. The scavenger studies indicated that the hydroxyl radicals (•OH) and holes (h+) active species are mainly responsible for the degradation of MB and MO dyes. The higher photocatalytic activities are attributed to the enhanced light absorption, increased adsorption capacity, and better charge separation in the Cu7S4 catalyst.
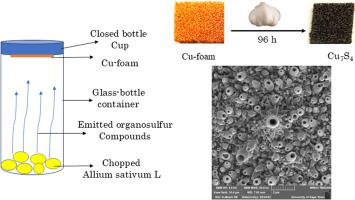
室温挥发性有机硫合成分级Cu7S4中空纳米管,用于光降解有机污染物
本研究开发了一种新颖、环保、可持续的绿色方法,利用大蒜的挥发性有机硫化合物在Cu泡沫上合成均匀中空Cu7S4纳米管,用于光催化有机污染物。这种新方法使泡沫铜在室温下无需表面活性剂(即无试剂)即可硫化,从而使泡沫铜具有独特的中空纳米管表面。采用扫描电镜(SEM)、x射线能谱(EDX)、x射线衍射(XRD)和x射线光电子能谱(XPS)对样品的形貌、组成、结构相和化学状态进行了分析。N2吸附-解吸等温线显示,Cu7S4中空纳米管的比表面积约为7.2 m2 g−1,孔径分布为46.89 nm。所得Cu7S4@Cu-foam用于可见光下降解亚甲基蓝(MB)和甲基橙(MO)污染物,表现出增强的光催化活性。MB和MO的准一级降解动力学速率分别为0.012和0.0098 min−1。清除剂研究表明,羟基自由基(•OH)和空穴活性物质(h+)对MB和MO染料的降解起主要作用。较高的光催化活性是由于Cu7S4催化剂的光吸收增强,吸附能力增强,电荷分离效果较好。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Solid State Sciences
化学-无机化学与核化学
CiteScore
6.60
自引率
2.90%
发文量
214
审稿时长
27 days
期刊介绍:
Solid State Sciences is the journal for researchers from the broad solid state chemistry and physics community. It publishes key articles on all aspects of solid state synthesis, structure-property relationships, theory and functionalities, in relation with experiments.
Key topics for stand-alone papers and special issues:
-Novel ways of synthesis, inorganic functional materials, including porous and glassy materials, hybrid organic-inorganic compounds and nanomaterials
-Physical properties, emphasizing but not limited to the electrical, magnetical and optical features
-Materials related to information technology and energy and environmental sciences.
The journal publishes feature articles from experts in the field upon invitation.
Solid State Sciences - your gateway to energy-related materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


