Caffeic acid-Fe3+ nanohybrids loaded with berberine/baicalin for NIR-activated antibacterial therapy via ROS generation and photothermal effects
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The escalating threat of bacterial infections underscores the pressing need for innovative antibacterial solutions. In this study, BA-BBR@MPN nanoparticles (BA-BBR@MPN NPs) were synthesized through the self-assembly of berberine (BBR) and baicalin (BA), followed by modification with a metal-phenolic network (MPN) derived from caffeic acid and Fe3+. In the slightly acidic environment of the infected site, the MPN decomposes to release iron ions, BBR, and BA. The iron ions catalyze the generation of hydroxyl radicals (·OH) from hydrogen peroxide (H2O2), enabling chemodynamic therapy (CDT) while simultaneously depleting glutathione levels. When combined with 808 nm laser-induced photothermal therapy (PTT), the system demonstrates synergistic antibacterial effects. Both in vitro and in vivo studies confirmed the platform's broad-spectrum antibacterial activity against Gram-positive and Gram-negative bacteria, along with excellent biocompatibility and significant wound-healing promotion. This multifunctional nanoplatform represents a promising strategy for combating bacterial infections while simultaneously promoting tissue repair.
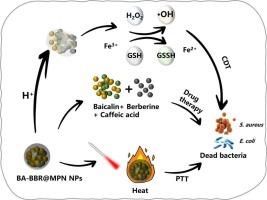
负载小檗碱/黄芩苷的咖啡酸- fe3 +纳米杂化体通过ROS生成和光热效应进行nir激活抗菌治疗
细菌感染威胁的不断升级凸显了创新抗菌解决方案的迫切需要。在这项研究中,通过小檗碱(BBR)和黄芩苷(BA)的自组装合成BA-BBR@MPN纳米粒子(BA-BBR@MPN NPs),然后用咖啡酸和Fe3+衍生的金属酚网络(MPN)修饰。在感染部位的微酸性环境中,MPN分解释放铁离子、BBR和BA。铁离子催化过氧化氢(H2O2)产生羟基自由基(·OH),使化学动力治疗(CDT)成为可能,同时消耗谷胱甘肽水平。当与808 nm激光诱导光热疗法(PTT)联合使用时,该系统显示出协同抗菌作用。体外和体内研究均证实该平台对革兰氏阳性和革兰氏阴性菌具有广谱抗菌活性,同时具有良好的生物相容性和显著的促进伤口愈合作用。这种多功能纳米平台代表了对抗细菌感染同时促进组织修复的有前途的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


