Ethyl transfer reactions of NaBEt4 with Cp*Rh(PMe3)PhBr and elimination reactions
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The reaction of NaBEt4 with Cp*Rh(PMe3)PhBr produces Cp*Rh(PMe3)PhEt by ethyl transfer. Single crystal X-ray analysis shows a typical piano-stool geometry. This compound reacts photochemically (λ > 320 nm) to give Cp*Rh(PMe3)PhH as the major product, plus ethylene. A secondary product was assigned as the square planar complex RhPh(PMe3)(η4-C5Me5Et) on the basis of NMR spectroscopy. The molecule decomposes over several days at ambient temperature, releasing C5Me5Et.
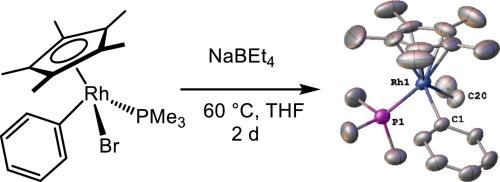
NaBEt4与Cp*Rh(PMe3)PhBr的乙基转移反应及消去反应
NaBEt4与Cp*Rh(PMe3)PhBr通过乙基转移反应生成Cp*Rh(PMe3)PhEt。单晶x射线分析显示典型的钢琴凳几何形状。该化合物光化学反应(λ > 320 nm)生成Cp*Rh(PMe3)PhH作为主要产物,外加乙烯。经核磁共振谱分析,二级产物为方平面配合物RhPh(PMe3)(η - 4- c5me5et)。这种分子在环境温度下分解几天,释放出C5Me5Et。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


