Kinetics of phosphine substitution in cyclopentadienylruthenium bis(triarylphosphine)chlorides CpRu(PAr3)2Cl (Ar = m-tolyl, m-fluorophenyl, m-anisyl, p-chlorophenyl) and Cp’Ru(PPh3)2Cl (Cp’ = CH3C5H4 and CH3C(O)C5H4)
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
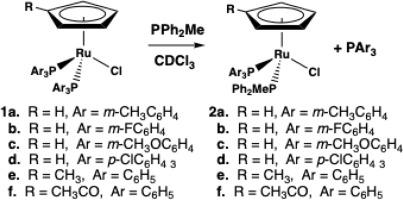
Reactions between CpRu(PAr3)2Cl (Ar = m-CH3C6H4 1a, m-FC6H4 1b, and m-CH3OC6H4 1c) and PMePh2 yield the corresponding mono-substituted products, CpRu(PAr3)(PMePh2)Cl (2a-c). Kinetic measurements for the reaction in CDCl3 under pseudo-first order conditions yield values for kobs ranging from 22.2±0.2 × 10-6 to 5.41±0.24 × 10–6 s–1. Activation parameters, ∆H† and ∆S†, range from 93±7 to 116±12 kJ/mol and –38±56 to 39±40 J/mol-K, respectively. The relative rates follow the order 1a > 1b > 1c. The rate constants for 1a-c are similar to kobs for CpRu(PAr3)2Cl where Ar = p-CH3C6H4 3a, p-FC6H4 3b, and p-CH3OC6H4 3c, suggesting that there is little or no steric effect of the m- vs p- position of the substituent on the Ar group. The rate of phosphine substitution in CpRu[P(p-ClC6H4)3]2Cl (1d) is slower than for 3a-3c. Despite different electronic properties (E1/2), similar rates are observed for reactions of (MeC5H4)Ru(PPh3)2Cl (1e) and (C5H4C(O)Me)Ru(PPh3)2Cl (1f) with PMePh2. Taken together, the data suggest that the rates of phosphine substitution in (η5-RC5H4)Ru(PAr3)2Cl complexes depends on a combination of σ-donor and π-acceptor properties of the PAr3 ligands.
环戊二烯基钌双(三芳基膦)氯化物CpRu(PAr3)2Cl (Ar = m- toyl, m- fluorphenyl, m-anisyl, p- chlorphenyl)和Cp ' ru (PPh3)2Cl (Cp ' = CH3C5H4和CH3C(O)C5H4)中的膦取代动力学
CpRu(PAr3)2Cl (Ar = m-CH3C6H4 1a, m-FC6H4 1b, m-CH3OC6H4 1c)与PMePh2反应生成相应的单取代产物CpRu(PAr3)(PMePh2)Cl (2a-c)。准一级条件下CDCl3反应的动力学测量,产率为22.2±0.2 × 10-6 ~ 5.41±0.24 × 10-6 s-1。活化参数∆H†和∆S†的范围分别为93±7 ~ 116±12 kJ/mol和-38±56 ~ 39±40 J/mol- k。相对速率的顺序为1a >; 1b > 1c。a-c的速率常数与Ar = p- ch3c6h4 3a、p- fc6h4 3b和p- ch3oc6h4 3c的CpRu(PAr3)2Cl的速率常数相似,表明Ar基上取代基的m-和p-位置几乎没有空间效应。CpRu[P(P - clc6h4)3]2Cl (1d)中的膦取代速率比3a-3c慢。尽管(MeC5H4)Ru(PPh3)2Cl (1e)和(C5H4C(O)Me)Ru(PPh3)2Cl (1f)与PMePh2的电子性质不同(E1/2),但反应速率相似。综上所述,(η5-RC5H4)Ru(PAr3)2Cl配合物中膦取代的速率取决于PAr3配体的σ-供体和π-受体性质的结合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


