M1 macrophages enhance breast cancer chemoresistance via JAK-STAT3 signaling
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-09-18
DOI:10.1016/j.bbadis.2025.168056
引用次数: 0
Abstract
Tumor-associated macrophages (TAMs) are the most abundant immune cells in the tumor microenvironment, playing a key role in breast cancer (BrCa) progression and chemotherapy response. While TAMs exhibit diverse phenotypes, the M1/M2 classification remains widely used. M1-like macrophages are known for tumor-killing properties, whereas M2-like macrophages promote tumor growth. However, the impact of TAM subtypes on chemotherapy response remains inconsistent. In this study, we found that M1-like macrophages or their conditioned medium (CM) induced greater BrCa cell death and inhibited proliferation compared to M2-like macrophages. Surprisingly, BrCa cells surviving M1-like macrophage-induced killing displayed increased chemotherapy resistance, independent of proliferation. Transcriptomic profiling indicated upregulation of the JAK–STAT signaling pathway, with elevated STAT3 phosphorylation subsequently confirmed at the protein level. Inhibition of JAKs with Ruxolitinib reduced STAT3 activation and restored chemotherapy sensitivity. Our findings highlight the dual role of M1-like macrophages, demonstrating both tumoricidal activity and the potential to induce chemotherapy resistance in surviving tumor cells, offering insights for macrophage-targeted therapies.
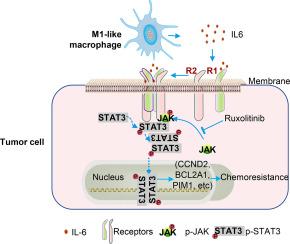
M1巨噬细胞通过JAK-STAT3信号增强乳腺癌化疗耐药。
肿瘤相关巨噬细胞(tumor -associated macrophages, tam)是肿瘤微环境中最丰富的免疫细胞,在乳腺癌的进展和化疗反应中起着关键作用。虽然tam表现出多种表型,但M1/M2分类仍被广泛使用。众所周知,m1样巨噬细胞具有肿瘤杀伤特性,而m2样巨噬细胞则促进肿瘤生长。然而,TAM亚型对化疗反应的影响仍不一致。在这项研究中,我们发现与m2样巨噬细胞相比,m1样巨噬细胞或其条件培养基(CM)诱导更大的乳腺癌(BrCa)细胞死亡并抑制增殖。令人惊讶的是,存活于m1样巨噬细胞诱导的杀伤中的BrCa细胞表现出增加的化疗耐药性,不依赖于增殖。RNA测序还揭示了这些癌细胞中JAK-STAT通路的上调和磷酸化STAT3的升高。Ruxolitinib抑制JAKs可降低STAT3的激活并恢复化疗敏感性。我们的研究结果强调了m1样巨噬细胞的双重作用,证明了在存活的肿瘤细胞中具有杀瘤活性和诱导化疗耐药的潜力,为巨噬细胞靶向治疗提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


