Lipid peroxidation-induced cell death in Rett syndrome
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Rett Syndrome (RTT) is a rare neurodevelopmental disorder, primarily affecting girls (1:10,000 live births), largely caused by mutations in the X-linked gene MECP2, an epigenetic regulator encoding for the methyl-CpG binding protein 2 (MeCP2). Recent evidence links ferroptosis, an iron-dependent cell death characterized by lipid peroxide accumulation, to neurodegenerative and neurodevelopmental disorders like autism.
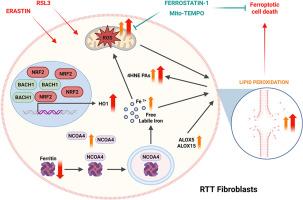
Several RTT hallmarks, including redox imbalance, excess labile iron, increased lipid peroxidation, and impaired antioxidant enzyme activity, align with ferroptosis characteristics. Therefore, we investigated ferroptosis's role in RTT using human primary fibroblasts from healthy and RTT subjects, treating them with ferroptosis inducers: erastin and RSL3.
Our findings show RTT cells are highly susceptible to ferroptosis, marked by elevated lipid peroxidation and mitochondrial reactive oxygen species (mtROS) production, crucial for ferroptotic cell death. We also observed altered iron metabolism and dysregulated ferritinophagy. RTT fibroblasts exhibited an imbalanced antioxidant defense, particularly after ferroptotic stimuli, and ferroptosis inducers worsened redox imbalance compared to controls. Importantly, a ferroptosis inhibitor (Ferrostatin-1) and a SOD mimetic (mito-TEMPO) prevented these effects and normalized the altered basal conditions of RTT cells.
In conclusion, our results reveal a general dysregulation in RTT cells contributing to increased ferroptosis sensitivity. This suggests a significant role for ferroptosis in RTT pathophysiology and progression, potentially opening new therapeutic avenues for this condition.
脂质过氧化诱导Rett综合征的细胞死亡。
Rett综合征(RTT)是一种罕见的神经发育障碍,主要影响女孩(1:10 000活产),主要由x连锁基因MECP2突变引起,MECP2是一种编码甲基- cpg结合蛋白2 (MECP2)的表观遗传调节因子。最近的证据表明,铁下垂(一种以脂质过氧化积累为特征的铁依赖性细胞死亡)与神经退行性和神经发育障碍(如自闭症)有关。RTT的几个特征,包括氧化还原失衡、不稳定铁过量、脂质过氧化增加和抗氧化酶活性受损,与铁下垂特征一致。因此,我们利用来自健康和RTT受试者的人原代成纤维细胞,用erastin和RSL3诱导剂处理它们,研究了铁凋亡在RTT中的作用。我们的研究结果表明,RTT细胞对铁下垂非常敏感,其特征是脂质过氧化和线粒体活性氧(mtROS)的产生升高,这对铁下垂细胞死亡至关重要。我们还观察到铁代谢改变和铁蛋白吞噬失调。RTT成纤维细胞表现出不平衡的抗氧化防御,特别是在铁沉刺激后,与对照组相比,铁沉诱导剂加剧了氧化还原不平衡。重要的是,一种ferroptosis抑制剂(Ferrostatin-1)和一种SOD模拟物(mito-TEMPO)阻止了这些影响,并使RTT细胞改变的基础条件正常化。总之,我们的结果揭示了RTT细胞的普遍失调导致铁下垂敏感性增加。这表明铁下垂在RTT的病理生理和进展中起着重要作用,可能为这种疾病开辟新的治疗途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


