Nrf2/Bach1 axis regulates redox homeostasis and energy metabolism to optimize Sertoli cell-mediated efferocytosis
IF 3.6
3区 医学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Efferocytosis is energy-consuming, and continuous efferocytosis imposes metabolic burdens on the phagocytes. Sertoli cells (SCs) are specialized phagocytes in the testis for efferocytosis of non-viable germ cells and residual bodies. What remains elusive is how SCs integrate metabolic adaptations in response to efferocytosis. Here, we identify the Nrf2/Bach1 axis as an important molecular machinery of SC-mediated efferocytosis. Nrf2 activation during efferocytosis stabilizes Bach1 expression. Nrf2 activation or Bach1 overexpression promotes SC-mediated efferocytosis, while the opposite phenotype is incurred by Nrf2 inactivation or Bach1 deficiency, with oxidative stress being a contributing factor. Beyond experiencing attenuated glucose uptake and ATP production, Bach1-deficient SCs exhibit a reduced NAD+/NADH ratio, and restraining NAD+ consumption by inhibiting serine biosynthesis rescues their impaired efferocytosis. We further observe an up-regulation of anti-ferroptotic genes in SCs upon Bach1 deficiency and demonstrate a protective role of ferroptosis in this scenario. We thus propose that redox homeostasis and energy metabolism lie at the nexus of the Nrf2/Bach1 axis in the regulation of SC-mediated efferocytosis. Our study explores the regulatory role of the Nrf2/Bach1 axis in SC-mediated efferocytosis, which will lead to a better appreciation of SCs in male reproductive health.
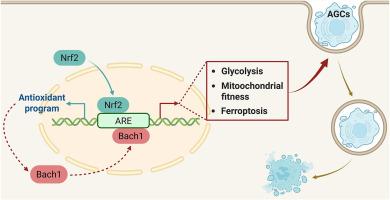
Nrf2/Bach1轴调节氧化还原稳态和能量代谢,优化支持细胞介导的efferocytosis。
Efferocytosis是消耗能量的,持续的Efferocytosis会给吞噬细胞带来代谢负担。支持细胞(SCs)是睾丸内的一种特化吞噬细胞,用于吞噬无活力的生殖细胞和残体。目前尚不清楚的是,SCs是如何在efferocytosis反应中整合代谢适应的。在这里,我们确定Nrf2/Bach1轴是sc介导的efferocytosis的重要分子机制。胞饮过程中Nrf2的激活稳定了Bach1的表达。Nrf2激活或Bach1过表达可促进sc介导的efferocytosis,而Nrf2失活或Bach1缺乏则会导致相反的表型,其中氧化应激是一个促成因素。除了葡萄糖摄取和ATP产生减弱外,bach1缺陷SCs还表现出NAD+/NADH比值降低,通过抑制丝氨酸生物合成来抑制NAD+消耗可以挽救受损的efferocytosis。我们进一步观察到Bach1缺乏时sc中抗铁沉基因的上调,并证明了在这种情况下铁沉的保护作用。因此,我们提出氧化还原稳态和能量代谢存在于Nrf2/Bach1轴在sc介导的efferocytosis调节中的联系。我们的研究探讨了Nrf2/Bach1轴在sc介导的efferocytosis中的调节作用,这将使我们更好地了解sc在男性生殖健康中的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular and Cellular Endocrinology
医学-内分泌学与代谢
CiteScore
9.00
自引率
2.40%
发文量
174
审稿时长
42 days
期刊介绍:
Molecular and Cellular Endocrinology was established in 1974 to meet the demand for integrated publication on all aspects related to the genetic and biochemical effects, synthesis and secretions of extracellular signals (hormones, neurotransmitters, etc.) and to the understanding of cellular regulatory mechanisms involved in hormonal control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


