Forskolin alleviates cholestatic liver disease by inhibiting the Hippo/YAP-mediated ductular reaction and fibrosis progression
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Cholestatic liver disease (CLD) results from impaired bile flow, involving dysregulated Hippo/YAP signaling driving pathogenic ductular reaction (DR), inflammation, and fibrosis. Although ursodeoxycholic acid (UDCA) remains first-line therapy, most patients show inadequate response. Our computational screening identified forskolin (FSK), adenylate cyclases (ADCY1–10) activator, as a potential Hippo/YAP pathway modulator through cAMP elevation. Molecular docking confirmed FSK's stable binding across all human ADCY isoforms, supporting upstream cAMP's activation. Cholestatic injury was induced in rats via bile duct ligation (BDL). BDL-rats were treated with either FSK or UDCA. Relative to UDCA, FSK significantly improved liver function (ALT, AST, ALP, GGT) and cholestasis (bilirubin, bile acids) parameters. FSK markedly reduced inflammation markers (IL-6, IL-1β, CXCL1, and CXCL2) and attenuated fibrosis, as evidenced by restored Hydroxyproline levels, COL1A1, and α-SMA expressions. Histological analysis revealed that FSK preserved hepatic architecture, suppressed DR, and downregulated CK19/EpCAM expressions, whereas UDCA showed only partial improvement. Although UDCA significantly modulated Hippo/YAP signaling, FSK substantially activated the pathway through LATS1/2 and YAP1 phosphorylation, suppressing YAP/TEAD transcriptional activity, mediated through cAMP elevation. These findings indicate FSK's multi-target therapeutic actions, mitigating inflammation, DR, and fibrosis mediated by ADCY-cAMP-Hippo/YAP axis modulation. Given its superior efficacy over UDCA, FSK represents a promising therapeutic candidate for clinical translation in CLD.
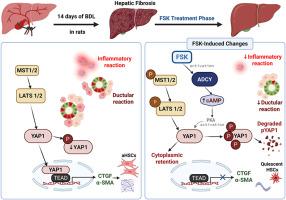
福斯可林通过抑制Hippo/ yap介导的导管反应和纤维化进展来缓解胆汁淤积性肝病。
胆汁淤积性肝病(CLD)由胆汁流动受损引起,涉及驱动致病性导管反应(DR)、炎症和纤维化的Hippo/YAP信号失调。虽然熊去氧胆酸(UDCA)仍然是一线治疗,但大多数患者表现出不足的反应。我们的计算筛选鉴定了腺苷酸环化酶(ADCY1-10)激活剂forskolin (FSK)通过cAMP升高作为潜在的Hippo/YAP通路调节剂。分子对接证实了FSK在所有人类ADCY亚型上的稳定结合,支持上游cAMP的激活。采用胆管结扎法(BDL)诱导大鼠胆汁淤积损伤。bdl大鼠分别给予FSK或UDCA治疗。与UDCA相比,FSK显著改善了肝功能(ALT、AST、ALP、GGT)和胆汁淤积(胆红素、胆汁酸)参数。FSK显著降低炎症标志物(IL-6、IL-1β、CXCL1和CXCL2)并减轻纤维化,羟脯氨酸水平、COL1A1和α-SMA表达均有所恢复。组织学分析显示,FSK保留了肝脏结构,抑制了DR,下调了CK19/EpCAM的表达,而UDCA仅显示了部分改善。尽管UDCA显著调节了Hippo/YAP信号,但FSK通过LATS1/2和YAP1磷酸化激活了该通路,通过cAMP升高介导抑制了YAP/TEAD的转录活性。这些发现表明FSK具有多靶点治疗作用,可减轻ADCY-cAMP-Hippo/YAP轴调节介导的炎症、DR和纤维化。鉴于其优于UDCA的疗效,FSK代表了CLD临床转化的有希望的治疗候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


