ZNF831-YTHDF1-DNMT1/3a feedback loop regulates lung carcinogenesis and progression through WNT7B-FZD5-β-catenin signalling axis
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Zinc finger protein 831 (ZNF831) is a typical transcription factor involved in gene expression regulation. However, its role and mechanism in lung cancer (LC) remain largely unknown. DNA methylation, hydroxymethylation, and RNA m6A modification were measured by MeDIP, hMeDIP, and MeRIP. The survival and prognostic value were identified using Kaplan-Meier and Cox regression analysis. The function effects, target molecules and signalling pathway were determined in cell and animal model. We found that ZNF831 expression was downregulated through DNA methylation during lung carcinogenesis. ZNF831 could improve survival rate and was an independent protective factor for LC patients. ZNF831 overexpression inhibited LC cell growth, invasion and migration. Conversely, ZNF831 knockdown led to opposite phenotype in vitro and in vivo. Mechanistically, ZNF831 inhibited the expression of RNA-binding protein YTHDF1 through transcriptional regulation and protein interaction. Importantly, YTHDF1 also inversely inhibited ZNF831 expression by promoting DNA methyltransferase DNMT1 and DNMT3a to induce DNA hypermethylation. In addition, ZNF831 inhibited tumor growth and progression through YTHDF1 mediated translational regulation of WNT pathway key genes WNT7B and FZD5. These results demonstrated that the feedback loop of ZNF831-YTHDF1-DNMT1/3a regulates cell growth, migration and invasion via WNT7B-FZD5-β-catenin axis, further providing a new idea for targeting epigenetic regulators of LC.
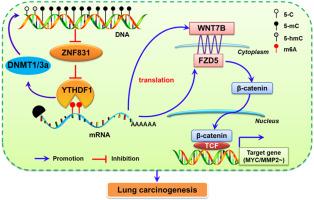
ZNF831-YTHDF1-DNMT1/3a反馈回路通过WNT7B-FZD5-β-catenin信号轴调控肺癌的发生和进展。
锌指蛋白831 (ZNF831)是参与基因表达调控的典型转录因子。然而,其在肺癌(LC)中的作用和机制在很大程度上仍然未知。通过MeDIP、hMeDIP和MeRIP检测DNA甲基化、羟甲基化和RNA m6A修饰。使用Kaplan-Meier和Cox回归分析确定生存和预后价值。在细胞和动物模型上测定其功能作用、靶分子和信号通路。我们发现ZNF831在肺癌发生过程中通过DNA甲基化下调。ZNF831可提高LC患者的生存率,是独立的保护因子。过表达ZNF831抑制LC细胞的生长、侵袭和迁移。相反,ZNF831敲低在体内和体外会导致相反的表型。机制上,ZNF831可以通过转录调控和蛋白相互作用抑制rna结合蛋白YTHDF1的表达。重要的是,YTHDF1还可以通过促进DNA甲基转移酶DNMT1和DNMT3a诱导DNA超甲基化来反向抑制ZNF831的表达。此外,ZNF831通过YTHDF1介导的WNT通路关键基因WNT7B和FZD5的翻译调节抑制肿瘤的生长和进展。这些结果表明,ZNF831-YTHDF1-DNMT1/3a的反馈回路通过WNT7B-FZD5-β-catenin轴调控细胞生长、迁移和侵袭,进一步为靶向LC的表观遗传调控因子提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


