The thiolation of U34 at carbon 2 in tRNA by Escherichia coli MnmA precedes modification at carbon 5 and is dependent on a [4Fe-4S] cluster
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
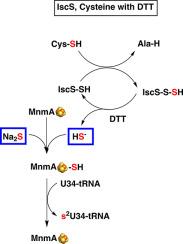
Chemical modifications of transfer RNAs (tRNAs) fine-tune the recognition of messenger RNA codons on the ribosome for accurate protein translation. Uridine 34 (U34) of the anticodon of three tRNAs is modified in all domains of life. In bacteria, U34 is modified at C5 by aminomethyl derivatives by MnmE/MnmG enzymes and at C2 by a sulfur atom by MnmA enzymes from two distinct classes. C-type MnmAs possess a CXXC+ C catalytic motif, in which cysteines bind a [4Fe-4S] cluster essential for catalysis. D-type MnmAs possess a DXXC+ C catalytic motif, which can also bind a [4Fe-4S] cluster, but the function of the cluster is controversial. To shed light on the role of the [4Fe-4S] cluster in the thiolation reaction, we report here new in vitro catalytic assays of the D-type Escherichia coli MnmA enzyme (EcMnmA) before, and after cluster reconstitution under anaerobic conditions. We confirm that that only holo-EcMnmA can thiolate U34 in a tRNA transcript using sulfide as a sulfur source and show that 1) a bulk E. coli ΔmnmA tRNA, containing the C5 modification, is not a substrate, indicating that thiolation at C2 precedes modification at C5, 2) cysteine with IscS cannot provide the sulfur atom for tRNA thiolation without a reductant and 3) the variant, in which the aspartate of the DXXC + C motif is replaced by cysteine, is efficient in U34-tRNA thiolation in vitro but not in vivo, raising questions about the in vivo function of soft cluster coordination.
大肠杆菌MnmA在tRNA中2号碳上对U34的硫基化先于5号碳上的修饰,并且依赖于[4Fe-4S]簇。
转运RNA (trna)的化学修饰微调了核糖体上信使RNA密码子的识别,以实现准确的蛋白质翻译。三种trna反密码子的尿嘧啶34 (U34)在生命的所有领域都被修饰。在细菌中,U34在C5处被MnmE/MnmG酶的氨基甲基衍生物修饰,在C2处被两个不同类别的MnmA酶的硫原子修饰。C型mnma具有CXXC+ C催化基序,其中半胱氨酸结合催化必需的[4Fe-4S]簇。d型mnma具有DXXC+ C催化基序,该基序也可以结合[4Fe-4S]簇,但簇的功能存在争议。为了阐明[4Fe-4S]簇在硫代化反应中的作用,我们在这里报道了d型大肠杆菌MnmA酶(EcMnmA)在厌氧条件下簇重组前后的体外催化分析。我们证实,只有holo-EcMnmA可以在使用硫化物作为硫源的tRNA转录物中硫化U34,并表明1)含有C5修饰的大肠杆菌ΔmnmA大量tRNA不是底物,表明C2的硫化先于C5的修饰,2)半胱氨酸与IscS不能在没有还原剂的情况下为tRNA硫化提供硫原子,3)DXXC + C基序的天门氨酸被半胱氨酸取代。在体外对U34-tRNA硫代化有效,但在体内无效,这就提出了软簇配合在体内功能的问题。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


