Intercellular communication interference through energy metabolism-related exosome secretion inhibition for liver fibrosis treatment
IF 14.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
As activated hepatic stellate cells (aHSCs) play a central role in fibrogenesis, they have become key target cells for anti-fibrotic treatment. Nevertheless, the therapeutic efficiency is constrained by the exosomes they secrete, which are linked to energy metabolism and continuously stimulate the activation of neighboring quiescent hepatic stellate cells (qHSCs). Herein, an intercellular communication interference strategy is designed utilizing paeoniflorin (PF) loaded and hyaluronic acid (HA) coated copper-doped ZIF-8 (PF@HA-Cu/ZIF-8, PF@HCZ) to reduce energy-related exosome secretion from aHSCs, thus preserving neighboring qHSCs in a quiescent state. Simultaneously, the released copper and zinc ions disrupt key enzymes involved in glycolysis to reduce bioenergy synthesis in aHSCs, thereby promoting the reversion of aHSCs to a quiescent state and further decreasing exosome secretion. Therefore, PF@HCZ can effectively sustain both aHSCs and qHSCs in a metabolically dormant state to ultimately alleviate liver fibrosis. The study provides an enlightening strategy for interrupting exosome-mediated intercellular communication and remodeling the energy metabolic status of HSCs with boosted antifibrogenic activity.
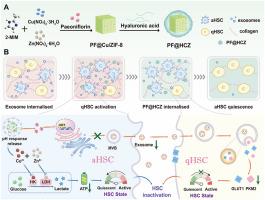
细胞间通讯干扰通过能量代谢相关外泌体分泌抑制肝纤维化治疗
由于活化的肝星状细胞(aHSCs)在纤维化发生中起着核心作用,它们已成为抗纤维化治疗的关键靶细胞。然而,治疗效率受到它们分泌的外泌体的限制,这些外泌体与能量代谢有关,并不断刺激邻近的静止肝星状细胞(qhsc)的激活。本文设计了一种细胞间通信干扰策略,利用负载芍药苷(PF)和透明质酸(HA)包被的铜掺杂ZIF-8 (PF@HA-Cu/ZIF-8, PF@HCZ)来减少aHSCs的能量相关外泌体分泌,从而使邻近的qhsc保持静止状态。同时,释放的铜离子和锌离子破坏参与糖酵解的关键酶,减少ahsc中生物能量的合成,从而促进ahsc恢复到静止状态,进一步减少外泌体的分泌。因此,PF@HCZ可以有效地维持aHSCs和qhsc处于代谢休眠状态,最终缓解肝纤维化。该研究为阻断外泌体介导的细胞间通讯和重塑hsc的能量代谢状态提供了一种具有启发性的策略,增强了抗纤维化活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Pharmaceutica Sinica. B
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
22.40
自引率
5.50%
发文量
1051
审稿时长
19 weeks
期刊介绍:
The Journal of the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association oversees the peer review process for Acta Pharmaceutica Sinica. B (APSB).
Published monthly in English, APSB is dedicated to disseminating significant original research articles, rapid communications, and high-quality reviews that highlight recent advances across various pharmaceutical sciences domains. These encompass pharmacology, pharmaceutics, medicinal chemistry, natural products, pharmacognosy, pharmaceutical analysis, and pharmacokinetics.
A part of the Acta Pharmaceutica Sinica series, established in 1953 and indexed in prominent databases like Chemical Abstracts, Index Medicus, SciFinder Scholar, Biological Abstracts, International Pharmaceutical Abstracts, Cambridge Scientific Abstracts, and Current Bibliography on Science and Technology, APSB is sponsored by the Institute of Materia Medica, Chinese Academy of Medical Sciences, and the Chinese Pharmaceutical Association. Its production and hosting are facilitated by Elsevier B.V. This collaborative effort ensures APSB's commitment to delivering valuable contributions to the pharmaceutical sciences community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


