Design and synthesis of 4-azaindoles derivatives: targeting the cardiac troponin I-interacting kinase (TNNI3K)
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Cardiac troponin I-interacting kinase (TNNI3K) is a cardiac-specific protein kinase, whose overexpression is closely linked to heart failure and ventricular remodeling. TNNI3K inhibitors regulate the phosphorylation of serine residues in downstream cardiac troponin I (cTnI) and affect the p38 pathway to prevent ventricular remodeling and myocardial cell damage. This study designed 120 compounds based on the reported quantitative structure-activity relationships (QSAR) of TNNI3K inhibitors. Following virtual screening, 4-azaindole was identified as the optimal scaffold. Subsequent synthesis of derivatives SK1–SK5 demonstrated their protective effects on damaged cardiomyocytes. Importantly, molecular dynamics (MD) simulations confirmed that compound SK5 forms a stable complex with TNNI3K and elucidated key binding residues and their interaction modes. These findings collectively validate the rational design of TNNI3K-targeted compounds and support SK5's potential as an anti-heart failure lead candidate.
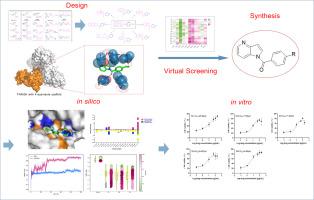
靶向心肌肌钙蛋白i相互作用激酶(TNNI3K)的4-氮唑衍生物的设计与合成
心肌肌钙蛋白i相互作用激酶(TNNI3K)是一种心脏特异性蛋白激酶,其过表达与心力衰竭和心室重构密切相关。TNNI3K抑制剂调节下游心肌肌钙蛋白I (cTnI)丝氨酸残基的磷酸化,影响p38通路,防止心室重构和心肌细胞损伤。本研究基于已报道的TNNI3K抑制剂的定量构效关系(QSAR)设计了120个化合物。经过虚拟筛选,4-叠氮都乐被确定为最佳支架。随后合成的衍生物SK1-SK5证明了它们对受损心肌细胞的保护作用。重要的是,分子动力学(MD)模拟证实了化合物SK5与TNNI3K形成稳定的配合物,并阐明了关键的结合残基及其相互作用模式。这些发现共同验证了tnni3k靶向化合物的合理设计,并支持SK5作为抗心力衰竭主要候选药物的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


