Mass spectrometric observation of a dipole-bound dimer anion [H2⋯H2O]− produced under atmospheric pressure Corona discharge: Formation mechanism and hyperconjugative stability
IF 3.1
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The formation of a hydrogen-included dipole-bound dimer anion, [H2⋯H2O]−, has been reported using a corona discharge mass spectrometer. Quantum chemical calculations suggested the hypothesis that the anion forms through an energetically favorable reaction, H− + H∙ + H2O → [H2⋯H2O]−. The calculated dipole moments of the [H2⋯H2O] complex range from 2.04 to 2.06 D, which may enable an electron to bind to the complex. A novel finding is that the Rydberg orbitals of the anion's NBO acceptor contribute to stabilizing the [H2⋯H2O]− anion by at least 15.37 kcal/mol (0.66 eV) through hyperconjugation.
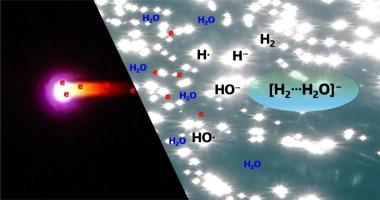
大气压下产生的偶极结合二聚阴离子[H2⋯H2O]−的质谱观察电晕放电:形成机制和超共轭稳定性
用电晕放电质谱仪报道了含氢偶极结合二聚阴离子[H2⋯H2O]−的形成。量子化学计算提出了阴离子通过能量有利反应H−+ H∙+ H2O→[H2⋯H2O]−形成的假设。计算的[H2⋯H2O]配合物的偶极矩范围为2.04至2.06 D,这可能使电子与配合物结合。一项新的发现是,阴离子的NBO受体的里德堡轨道通过超共轭作用有助于稳定[H2⋯H2O]−阴离子至少15.37 kcal/mol (0.66 eV)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics Letters
化学-物理:原子、分子和化学物理
CiteScore
5.70
自引率
3.60%
发文量
798
审稿时长
33 days
期刊介绍:
Chemical Physics Letters has an open access mirror journal, Chemical Physics Letters: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Chemical Physics Letters publishes brief reports on molecules, interfaces, condensed phases, nanomaterials and nanostructures, polymers, biomolecular systems, and energy conversion and storage.
Criteria for publication are quality, urgency and impact. Further, experimental results reported in the journal have direct relevance for theory, and theoretical developments or non-routine computations relate directly to experiment. Manuscripts must satisfy these criteria and should not be minor extensions of previous work.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


