Radical scavenging mechanism of 1H-benzimidazole-2-yl hydrazones and kinetics with physiologically relevant radicals: A computational study
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
A detailed computational study was performed to explain the relationship between the experimentally established radical scavenging ability of 1H-benzimidazol-2-yl hydrazones and their structural properties. Two dihydroxypheyl substituted compounds with high antioxidant activity and one substantially less active dimethoxyphenyl derivative were studied in benzene and water, mimicking the nonpolar and polar biological medium. The possibility for different reaction pathways was evaluated by Gibbs free energies of possible reactions, respective transition states and rate constants with •ОСН3, •OOH and •ОOСН3. It was found that in nonpolar medium, deactivation of free radicals would occur only by HAT mechanism. In water, deactivation of free radicals would proceed mainly by SET after deprotonation. In both solvents, the three compounds are able to deactivate •OOCH3 and stop the propagation of the lipid peroxidation reaction. The calculated overall rate coefficients showed that the 3,4-dihydroxy derivative is the most reactive one against all studied free radicals.
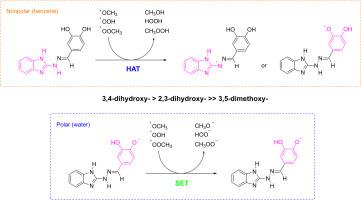
1h -苯并咪唑-2-酰基腙清除自由基的机制和生理相关自由基的动力学:计算研究
通过详细的计算研究来解释1h -苯并咪唑-2-酰基腙的自由基清除能力与其结构性质之间的关系。在苯和水中模拟非极性和极性生物介质,研究了两种具有高抗氧化活性的二羟基取代化合物和一种活性较低的二甲氧基苯基衍生物。通过反应的吉布斯自由能、过渡态和与•ОСН3、•OOH和•ОOСН3的反应速率常数来评价不同反应途径的可能性。发现在非极性介质中,自由基的失活仅通过HAT机制发生。在水中,脱质子后自由基的失活主要通过SET进行。在两种溶剂中,这三种化合物都能使•OOCH3失活并阻止脂质过氧化反应的进行。计算的总速率系数表明,3,4-二羟基衍生物对所有自由基的反应最活跃。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


