Design and synthesis of novel quinoline-chalcone derivatives as dual inhibitors of tubulin polymerization and P-glycoprotein to overcome cisplatin resistance in cervical cancer
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Cervical cancer remains a major cause of cancer death in women, often limited by cisplatin resistance. To overcome multidrug resistance (MDR), we designed and synthesized 23 novel quinoline-chalcone derivatives targeting both P-glycoprotein (P-gp) and the colchicine-binding site (CBS) of tubulin. Among them, compound 6h exhibited the most potent anti-proliferative activity against both cisplatin-sensitive HeLa cells (IC₅₀ = 6.69 μM) and cisplatin-resistant HeLa/DDP cells (IC₅₀ = 7.21 μM). The potency of compound 6h was superior than cisplatin (HeLa: 15.54 μM; HeLa/DDP: 94.32 μM) while displaying lower cytotoxicity towards normal cervical cells than cisplatin. Compound 6h demonstrated lower cisplatin resistance index (RI) in the HeLa/DDP cells than verapamil (RI: 1.27 vs. 2.22). Mechanistic studies demonstrated that compound 6h inhibited tubulin polymerization and induced G₂/M arrest and apoptosis in both the cell lines. Compound 6h reversed MDR by inhibiting P-gp efflux function, as evidenced by rhodamine 123 accumulation in HeLa/DDP cells. Molecular docking and dynamics simulations provided structural insights, confirming stable binding of 6h to the tubulin CBS (ΔG = −12.4 kcal/mol) and the P-gp hydrophobic lumen (ΔG = −10.8 kcal/mol). Zebrafish acute toxicity assay results demonstrated that the safety profile of compound 6h (0 % mortality at 400 μM) was superior than cisplatin (16.7 % mortality rate at 8 μM). To the best of our knowledge, compound 6h is the first reported quinoline-chalcone derivative with dual functions: direct antitumor activity and reversal of P-gp-mediated cisplatin resistance. Our results suggest that compound 6h is a promising compound and represents a novel strategy for combating drug-resistant cervical cancer.
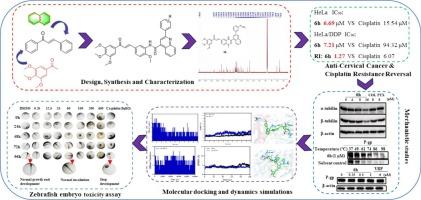
新型喹啉-查尔酮衍生物作为微管蛋白聚合和p -糖蛋白双重抑制剂克服宫颈癌顺铂耐药的设计与合成
宫颈癌仍然是妇女癌症死亡的主要原因,通常受到顺铂耐药性的限制。为了克服多药耐药(MDR),我们设计并合成了23种新的喹啉查尔酮衍生物,它们同时靶向p -糖蛋白(P-gp)和微管蛋白的秋水仙碱结合位点(CBS)。其中,化合物6h对顺铂敏感的HeLa细胞(IC₅₀= 6.69 μM)和顺铂抗性的HeLa/DDP细胞(IC₅₀= 7.21 μM)均表现出最有效的抗增殖活性。化合物6h效价优于顺铂(HeLa: 15.54 μM; HeLa/DDP: 94.32 μM),对正常宫颈细胞的细胞毒性低于顺铂。化合物6h在HeLa/DDP细胞中的顺铂耐药指数(RI)低于维拉帕米(RI: 1.27 vs. 2.22)。机制研究表明,化合物6h抑制微管蛋白聚合,诱导两种细胞系的G₂/M阻滞和凋亡。化合物6h通过抑制P-gp外排功能逆转MDR,这一点通过罗丹明123在HeLa/DDP细胞中的积累得到证实。分子对接和动力学模拟提供了结构上的见解,证实了6h与微管蛋白CBS (ΔG =−12.4 kcal/mol)和P-gp疏水管腔(ΔG =−10.8 kcal/mol)的稳定结合。斑马鱼急性毒性试验结果表明,化合物6h的安全性(400 μM的死亡率为0%)优于顺铂(8 μM的死亡率为16.7%)。据我们所知,化合物6h是第一个报道的具有双重功能的喹啉-查尔酮衍生物:直接抗肿瘤活性和逆转p- gp介导的顺铂耐药。我们的研究结果表明,化合物6h是一种很有前途的化合物,代表了一种对抗耐药宫颈癌的新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


