Curcumin protects against PIICS-induced liver injury by suppressing iron-induced lipid peroxidation: Insights from network pharmacology and experimental validation
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-09-20
DOI:10.1016/j.bbadis.2025.168053
引用次数: 0
Abstract
Background
Persistent inflammation, immunosuppression, and catabolism syndrome (PIICS) drives severe metabolic dysregulation and hepatic injury, characterized by hepatocyte damage and fibrosis. While curcumin (CUR) exhibits hepatoprotective properties, its role in PIICS-induced liver injury remains unexplored.
Methods
This study investigates the efficacy and mechanisms of CUR against PIICS-induced hepatic damage. Murine PIICS models underwent hepatic histopathology (H&E staining, TEM), RNA sequencing, and targeted metabolomics to identify injury markers. CUR's effects were assessed via biochemical, transcriptomic, and metabolomic analyses. Network pharmacology, molecular docking, and qPCR were employed to validate CUR targets.
Results
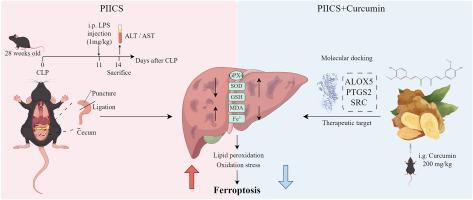
PIICS mice exhibited elevated serum ALT, AST, IL-6, and TNF-α levels, alongside histopathological evidence of inflammatory infiltration. Transcriptomics revealed dysregulation in lipid metabolism, redox homeostasis, and iron-binding pathways. Metabolomics identified hepatic polyunsaturated fatty acid (PUFA) depletion, increased ferrous ions, lipid peroxidation (4-HNE, MDA), and reduced antioxidants (GSH, SOD). CUR treatment alleviated liver injury, restored redox balance, and suppressed ferroptosis markers. Integrated analyses demonstrated that CUR targets ferroptosis-related pathways, modulating lipid metabolism, iron homeostasis, and oxidative stress.
Conclusion
CUR mitigates PIICS-induced liver injury by counteracting ferroptosis through modulation of lipid metabolism, iron homeostasis, and oxidative stress, offering a promising therapeutic strategy.
姜黄素通过抑制铁诱导的脂质过氧化来保护piics诱导的肝损伤:来自网络药理学和实验验证的见解
背景:持续的炎症、免疫抑制和分解代谢综合征(PIICS)会导致严重的代谢失调和肝损伤,其特征是肝细胞损伤和纤维化。虽然姜黄素(CUR)具有保护肝脏的特性,但其在piics诱导的肝损伤中的作用尚不清楚。方法研究CUR抗piics肝损伤的疗效及机制。小鼠PIICS模型通过肝脏组织病理学(H&;E染色,TEM), RNA测序和靶向代谢组学来鉴定损伤标志物。通过生化、转录组学和代谢组学分析评估CUR的效果。采用网络药理学、分子对接、qPCR等方法对CUR靶点进行验证。结果spics小鼠血清ALT、AST、IL-6和TNF-α水平升高,伴有炎症浸润的组织病理学证据。转录组学揭示了脂质代谢、氧化还原稳态和铁结合途径的失调。代谢组学鉴定肝脏多不饱和脂肪酸(PUFA)耗竭,亚铁离子增加,脂质过氧化(4-HNE, MDA)和抗氧化剂(GSH, SOD)减少。CUR治疗可减轻肝损伤,恢复氧化还原平衡,抑制铁下垂标志物。综合分析表明,CUR靶向与铁中毒相关的途径,调节脂质代谢、铁稳态和氧化应激。结论cur通过调节脂质代谢、铁稳态和氧化应激,抑制铁下沉,减轻piics诱导的肝损伤,是一种很有前景的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


