Thermally stable K2ZnP2O7: Eu3+ red emitting phosphor for w-LEDs
IF 2.6
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
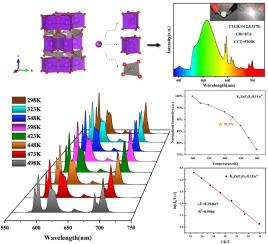
Red phosphors with good luminescent properties have attracted widespread attention because they can satisfy the lighting requirement of white LEDs (Light Emitting Diode) preferably. K2ZnP2O7: Eu3+ powder with a stable structure was successfully synthesized via the high-temperature solid-state method, involving a two-step process: pre-sintering at 450 °C for 12 h followed by sintering at 650 °C for 8 h. Under the excitation of 394 nm near ultraviolet light, the phosphor produces bright red emission. The highest emission peak of the K2Zn(1–1.5x)P2O7: xEu3+ (x = 0.1) phosphor is located at 595 nm (5D0 → 7F1), followed by the red emission peak at 612 nm (5D0 → 7F2). When the temperature reaches 150 °C, the emission integral intensity of K2ZnP2O7: 0.1Eu3+ remains 91.1% of that at room temperature (ΔE = 0.1946 eV), which proves that K2ZnP2O7: Eu3+ has good thermal stability. In addition, the CIE (International Commission on Illumination) coordinates of the white LED packaged by K2ZnP2O7: 0.1Eu3+ are (0.3312,0.3578), and the CCT (Correlated Color Temperature) and CRI (Color Rendering Index) of the LED lamp are 5765 K and 87.6, respectively. This indicates that K2ZnP2O7:Eu3+ is promised as a red-emitting phosphor for white LED excited by near-ultraviolet chips.
热稳定的K2ZnP2O7: Eu3+发光荧光粉
红色荧光粉具有良好的发光性能,能较好地满足白光led的照明要求,引起了人们的广泛关注。采用高温固相法成功合成了结构稳定的K2ZnP2O7: Eu3+粉末,共分为两步:450℃预烧结12 h, 650℃烧结8 h。在394 nm近紫外光激发下,荧光粉产生亮红色发光。K2Zn(1-1.5x)P2O7: xEu3+ (x = 0.1)荧光粉的最高发射峰位于595 nm处(5D0→7F1),其次是612 nm处(5D0→7F2)的红色发射峰。当温度达到150℃时,K2ZnP2O7: 0.1Eu3+的发射积分强度保持在室温时的91.1% (ΔE = 0.1946 eV),证明K2ZnP2O7: Eu3+具有良好的热稳定性。此外,由K2ZnP2O7: 0.1Eu3+封装的白光LED的CIE(国际照明委员会)坐标分别为(0.3312,0.3578),LED灯的CCT(相关色温)和CRI(显色指数)分别为5765 K和87.6。这表明K2ZnP2O7:Eu3+有望作为近紫外芯片激发的白光LED的红色发光荧光粉。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


