Sepsis-induced lipid droplet accumulation enhances antibacterial innate immunity through mechanisms dependent on DGAT-1 and interferon-beta
IF 11.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Lipid droplets (LDs) are lipid-rich organelles recognized as central players in lipid homeostasis, signaling, and inflammation. While their functions in inflammation are well-documented, the mechanisms of LDs in antibacterial immunity and infection resistance remain less understood. Our results show that E. coli-infection trigger immunometabolic reprogramming and LD accumulation in murine macrophages (BMDM). Moreover, purified LDs from LPS-stimulated and E. coli-infected macrophages exhibited direct E. coli anti-bacterial activity. Pharmacological inhibition or genetic knockdown of DGAT1, a key enzyme in triglyceride synthesis, reduced LD formation, bacterial clearance, and pro-inflammatory responses (nitric oxide, PGE2, CCL2, IL-6). Notably, DGAT1 inhibition impaired the expression of IFN-β and interferon-stimulated genes (ISGs), including viperin, iNOS, cathelicidin and IGTP, in E. coli-infected macrophages. In a cecal-ligation and puncture model of sepsis in C57BL/6 mice, DGAT1 inhibition reduced sepsis-induced LD accumulation in peritoneal cells and decreased levels of IFN-β, CCL2, nitric oxide, and lipid mediators (PGE2, LTB4, and RvD1) in the peritoneum. Furthermore, DGAT1 inhibition accelerated sepsis-related mortality, coinciding with elevated bacterial loads in the peritoneum and bloodstream at 6- and 24-h post-sepsis. Our results demonstrate that LDs are critical regulators of innate immunity infection resistance, contributing to both bacterial clearance and the coordination of a protective proinflammatory response during sepsis through mechanisms dependent on DGAT-1 and Type I IFN.
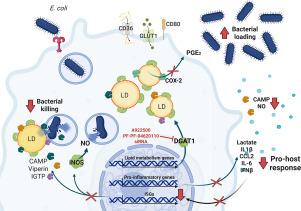
脓毒症诱导的脂滴积累通过依赖于DGAT-1和干扰素- β的机制增强抗菌先天免疫。
脂滴(ld)是一种富含脂质的细胞器,被认为是脂质稳态、信号传导和炎症的中心角色。虽然它们在炎症中的功能已被充分证明,但lld在抗菌免疫和感染抵抗中的机制仍不太清楚。我们的研究结果表明,大肠杆菌感染引发巨噬细胞的免疫代谢重编程和LD积累。此外,从lps刺激和大肠杆菌感染的巨噬细胞中纯化的ld显示出直接的大肠杆菌抗菌活性。DGAT1是甘油三酯合成、减少LD形成、细菌清除和促炎反应(一氧化氮、PGE2、CCL2、IL-6)的关键酶,其药理抑制或基因敲低。值得注意的是,DGAT1抑制抑制了大肠杆菌感染巨噬细胞中IFN-β和几种干扰素刺激基因(ISGs)的表达,包括viperin、iNOS、cathelicidin和IGTP。在C57BL/6小鼠脓毒症的盲肠结扎和穿刺模型中,DGAT1抑制降低了脓毒症诱导的腹膜细胞LD积累,降低了IFN-β、CCL2、一氧化氮和脂质介质(PGE2、LTB4和RvD1)的水平。此外,DGAT1抑制加速败血症相关死亡率,与败血症后6和24小时腹膜和血液中细菌负荷升高相一致。我们的研究结果表明,ld是先天免疫感染抵抗的关键调节因子,通过依赖于DGAT-1和I型IFN的机制,在败血症期间促进细菌清除和保护性促炎反应的协调。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Metabolism: clinical and experimental
医学-内分泌学与代谢
CiteScore
18.90
自引率
3.10%
发文量
310
审稿时长
16 days
期刊介绍:
Metabolism upholds research excellence by disseminating high-quality original research, reviews, editorials, and commentaries covering all facets of human metabolism.
Consideration for publication in Metabolism extends to studies in humans, animal, and cellular models, with a particular emphasis on work demonstrating strong translational potential.
The journal addresses a range of topics, including:
- Energy Expenditure and Obesity
- Metabolic Syndrome, Prediabetes, and Diabetes
- Nutrition, Exercise, and the Environment
- Genetics and Genomics, Proteomics, and Metabolomics
- Carbohydrate, Lipid, and Protein Metabolism
- Endocrinology and Hypertension
- Mineral and Bone Metabolism
- Cardiovascular Diseases and Malignancies
- Inflammation in metabolism and immunometabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


