A novel cholesterol-reducing mechanism of polygonati rhizoma: Dual action via Bacteroides-mediated cholesterol sulfonation and feedback inhibition of ACAT2 by sulfated metabolite
IF 5.4
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Ethnopharmacological relevance
Polygonati Rhizoma (PR) has the function of “invigorating spleen and tonifying kidney”, and is historically applied as a homology of medicine and food to prevent and treat dyslipidemia in China. However, there is limited experimental evidence to support this application, and the underlying mechanism has not been fully deciphered.
Aim of the study
To analyze the composition and illuminate the cholesterol-lowering potential and molecular mechanism of PR's aqueous extract (PRE) in high-fat emulsion (HFE)-induced hypercholesterolemia mouse model.
Materials and methods
Ion chromatograph was employed to determine the monosaccharide composition of PRE. HFE-induced Kunming mouse model was constructed to evaluate the anti-hypercholesterolemia effect of PRE. Metagenomic sequences and liquid chromatography-mass spectrometry (LC-MS) analysis were performed to elucidate the mechanism through which PR regulated cholesterol metabolism. Antibiotic cocktail (ABX) intervention and fecal microbiota transplantation (FMT) were used to validate whether PRE regulated cholesterol metabolism through the intestinal microbiota. The cholesterol-reducing effect of cholesterol sulfate (CS) was explored in poloxamer 407 (P407)-induced mouse model of dyslipidemia. Molecular docking and molecular dynamics (MD) simulation were also employed to elucidate the underlying mechanisms. Furthermore, a combination of qRT-PCR, Western blot, and surface plasmon resonance (SPR) were employed to delineate its mechanism.
Results
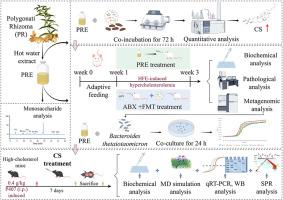
Our study indicated that the polysaccharides of PRE were mainly composed of fructose (92.33 %) and glucose (5.25 %). PRE treatment effectively blocked body weight gain, significantly decreased serum and hepatic levels of triglycerides (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C), and increased high-density lipoprotein cholesterol (HDL-C) level. Additionally, PRE ameliorated hepatic lipid accumulation in mice with HFE-elicited hypercholesterolemia. Notably, metagenomic sequencing and LC-MS analysis indicated that PRE markedly increased the abundance of intestinal genera Bacteroides and significantly elevated the fecal CS concentration in HFE mice. Genome-based functional analysis further indicated that cofactors of sulfonation (ATP sulfurylase CysD and CysN, BT0414-BT0415) were significantly upregulated after treatment with PRE. The cholesterol-lowering effect of PRE was largely contingent upon microbial conversion of cholesterol-to-CS mediated by Bacteroides, as validated by antibiotics-induced intestinal microbiota depletion in pseudo-germ-free model and restoration of gut microbiota through FMT. In vitro study also showed that PRE promoted the growth of Bacteroides thetaiotaomicron. Furthermore, CS markedly alleviated serum, hepatic, bile, and fecal levels of TG, TC, LDL-C, HDL-C, and TBA, indicative of appreciable lipid-lowering effect. MD simulation and SPR results indicated that CS directly bound to ACAT2. Consistent with this interaction, CS greatly downregulated the mRNA and protein expression of ACAT2 in small intestinal tissue.
Conclusion
These findings for the first time suggested that PR acted as a prebiotic agent to ameliorate hypercholesterolemia, at least in part, via dual mechanism involving modulation of Bacteroides-mediated sulfonation metabolic pathway and feedback inhibition of ACAT2 by CS, highlighting its therapeutic potential for cholesterol-related disorders. This work might also offer novel mechanistic insight and further buttressed the ethnopharmacological application of PR in the therapy of hypercholesterolemia.
黄精降胆固醇的新机制:拟杆菌介导的胆固醇磺化和硫酸代谢物对ACAT2的反馈抑制双重作用。
民族药理学相关性:黄精具有“健脾补肾”的功效,在中国历来作为药食同源物用于防治血脂异常。然而,有有限的实验证据来支持这一应用,并没有完全破译潜在的机制。目的:通过对高脂乳剂(HFE)诱导的高胆固醇血症小鼠模型的组成分析,阐明其降胆固醇潜能及分子机制。材料与方法:采用离子色谱法测定PRE的单糖组成。通过构建hfe诱导昆明小鼠模型,揭示PRE抗高胆固醇血症的作用。通过宏基因组测序和液相色谱-质谱(LC-MS)分析来阐明PR调节胆固醇代谢的机制。使用抗生素鸡尾酒(ABX)干预和粪便微生物群移植(FMT)来验证PRE是否通过肠道微生物群调节胆固醇代谢。在poloxam407 (P407)诱导的血脂异常小鼠模型中,探讨了硫酸胆固醇(CS)的降胆固醇作用。分子对接和分子动力学(MD)模拟也被用来阐明潜在的机制。此外,采用qRT-PCR、Western blot和表面等离子体共振(SPR)相结合的方法来描述其机制。结果:我们的研究表明,PRE多糖主要由果糖(92.33%)和葡萄糖(5.25%)组成。预处理能有效阻断体重增加,显著降低血清和肝脏甘油三酯(TG)、总胆固醇(TC)、低密度脂蛋白胆固醇(LDL-C)水平,提高高密度脂蛋白胆固醇(HDL-C)水平。此外,PRE还能改善hfe诱导的高胆固醇血症小鼠的肝脏脂质积累。宏基因组测序和LC-MS分析显示,PRE显著增加了HFE小鼠肠道拟杆菌属的丰度,显著提高了粪便CS浓度。基于基因组的功能分析进一步表明,磺化辅助因子(ATP硫酰化酶CysD和CysN, BT0414-BT0415)在PRE治疗后显著上调。PRE的降胆固醇作用在很大程度上取决于拟杆菌介导的胆固醇到cs的微生物转化,这一点通过抗生素诱导的伪无菌模型肠道微生物群消耗和通过FMT恢复肠道微生物群得到了验证。体外研究还表明,PRE促进了拟杆菌的生长。此外,CS显著降低了血清、肝脏、胆汁和粪便中TG、TC、LDL-C、HDL-C和TBA的水平,表明具有明显的降脂作用。MD模拟和SPR结果表明,CS直接与ACAT2结合。与这种相互作用一致,CS显著下调小肠组织中ACAT2 mRNA和蛋白的表达。结论:这些研究结果首次表明,PR作为一种益生元药物,至少在一定程度上通过调节拟杆菌介导的磺化代谢途径和CS对ACAT2的反馈抑制的双重机制来改善高胆固醇血症,突出了其治疗胆固醇相关疾病的潜力。这项工作也可能提供新的机制见解,并进一步支持PR在治疗高胆固醇血症中的民族药理学应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of ethnopharmacology
医学-全科医学与补充医学
CiteScore
10.30
自引率
5.60%
发文量
967
审稿时长
77 days
期刊介绍:
The Journal of Ethnopharmacology is dedicated to the exchange of information and understandings about people''s use of plants, fungi, animals, microorganisms and minerals and their biological and pharmacological effects based on the principles established through international conventions. Early people confronted with illness and disease, discovered a wealth of useful therapeutic agents in the plant and animal kingdoms. The empirical knowledge of these medicinal substances and their toxic potential was passed on by oral tradition and sometimes recorded in herbals and other texts on materia medica. Many valuable drugs of today (e.g., atropine, ephedrine, tubocurarine, digoxin, reserpine) came into use through the study of indigenous remedies. Chemists continue to use plant-derived drugs (e.g., morphine, taxol, physostigmine, quinidine, emetine) as prototypes in their attempts to develop more effective and less toxic medicinals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


