USP11 stabilizes RALY to promote FXYD5-mediated aerobic glycolysis and aggravate pancreatic cancer progression
IF 3.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
Background
Pancreatic cancer (PC) is a highly malignant and aggressive gastrointestinal malignancy with a poor prognosis for patients. Aerobic glycolysis serves as a critical metabolic driver of PC progression. Our study aims to elucidate the mechanism by which FXYD5 regulates aerobic glycolysis in PC.
Methods
Cell viability was assessed using CCK-8. Cell proliferation was tested by colony formation assay. Lactic acid production and ATP levels were measured by commercial kits. Extracellular acidification rate (ECAR) was detected using the Seahorse XF96 analyzer. RNA immunoprecipitation (RIP) was employed to validate the binding of RALY to FXYD5 mRNA. The interaction between RALY and USP11, as well as the ubiquitinated level of RALY, were probed by co-immunoprecipitation (Co-IP) assay. A subcutaneous xenograft tumor model was established to verify the in vitro findings.
Results
Knockdown of FXYD5 suppressed lactic acid production, ECAR, ATP levels, and the expression of aerobic glycolysis-related key markers (GLUT1 and HK2) in PC cells. RALY directly bound to FXYD5 mRNA to promote its stability in PC cells. RALY facilitated aerobic glycolysis to increase PC tumor growth by up-regulating FXYD5 expression in vitro and in vivo. Deubiquitinating enzyme USP11 reduced the ubiquitinated level of RALY to maintain its protein stability. USP11 knockdown restrained aerobic glycolysis of PC cells, which was reversed by RALY overexpression.
Conclusion
USP11 stabilized FXYD5 mRNA by promoting RALY deubiquitinated modification to increase PC cell aerobic glycolysis and exacerbate tumor progression.
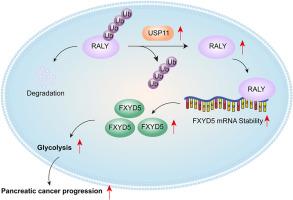
USP11稳定RALY促进fxyd5介导的有氧糖酵解并加重胰腺癌进展。
背景:胰腺癌是一种高度恶性、侵袭性的胃肠道恶性肿瘤,预后较差。有氧糖酵解是PC进展的关键代谢驱动因素。我们的研究旨在阐明FXYD5调控PC有氧糖酵解的机制。方法:采用CCK-8法测定细胞活力。用集落形成法检测细胞增殖。乳酸生成和ATP水平用商用试剂盒测定。采用Seahorse XF96分析仪检测细胞外酸化速率(ECAR)。采用RNA免疫沉淀法(RIP)验证RALY与FXYD5 mRNA的结合。采用共免疫沉淀(Co-IP)法检测RALY与USP11的相互作用以及RALY的泛素化水平。建立皮下异种移植瘤模型以验证体外实验结果。结果:FXYD5敲低可抑制PC细胞乳酸生成、ECAR、ATP水平以及好氧糖酵解相关关键标志物(GLUT1和HK2)的表达。RALY直接与FXYD5 mRNA结合,促进其在PC细胞中的稳定性。RALY通过上调FXYD5在体外和体内的表达,促进有氧糖酵解,促进PC肿瘤生长。去泛素化酶USP11降低了RALY的泛素化水平,以维持其蛋白稳定性。USP11敲低抑制了PC细胞的有氧糖酵解,这一作用被RALY过表达逆转。结论:USP11通过促进RALY去泛素化修饰,稳定FXYD5 mRNA,增加PC细胞有氧糖酵解,加速肿瘤进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


