Circadian rhythm disturbance induces osteoarthritis in mice: Involvement of Clock and Bmal1 dysregulation and Pdgfa inhibition
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Circadian rhythm disturbance (CRD) is likely associated with impaired bone development and the development of osteoarthritis (OA). This study investigates the functions of circadian locomotor output cycles kaput (Clock) and brain and muscle Arnt-like 1 (Bmal1) in OA progression and explores the implicated molecular mechanisms. Mice were subjected to CRD for 70 d. This led to significant OA-like symptoms, accompanied by a reduction in the expression of Clock and Bmal1. Either Clock or Bmal1 upregulation reduced serum concentrations of inflammatory cytokines (interleukin [IL]-1β and IL-6) in mice, and it reduced cartilage erosion and extracellular matrix degradation in their knee joints, with parallel findings observed in the isolated chondrocytes in vitro. The Clock:Bmal1 heterodimer binds to the enhancer upstream of the Pdgfa promoter to enhance its transcription. Pdgfa knockdown reversed the protective effects of Clock and Bmal1 by inactivating the Pi3k/Akt and Erk1/2 cascade. By contrast, Pdgfa overexpression reduced chondrocyte damage, which was, however, negated by the Pi3k/Akt inhibitor LY294002. Collectively, this study validates that CRD is pertinent to the development of OA. Restoring Clock and Bmal1 levels may help ameliorate chondrocyte loss in CRD-associated OA by activating Pdgfa transcription and enhancing Pi3k/Akt and Erk1/2 signaling pathways.
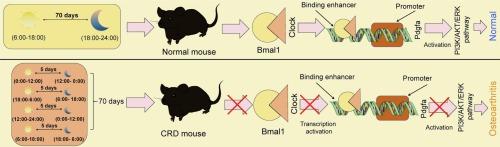
昼夜节律紊乱诱导小鼠骨关节炎:参与时钟和Bmal1失调和Pdgfa抑制。
昼夜节律紊乱(CRD)可能与骨骼发育受损和骨关节炎(OA)的发展有关。本研究探讨了昼夜运动输出周期kaput (Clock)和脑肌类阿氏蛋白样1 (Bmal1)在骨性关节炎进展中的作用,并探讨了相关的分子机制。小鼠接受CRD治疗70 d,导致明显的oa样症状,并伴有Clock和Bmal1表达的降低。Clock或Bmal1上调均可降低小鼠血清中炎症细胞因子(白细胞介素[IL]-1β和IL-6)的浓度,并减少膝关节软骨侵蚀和细胞外基质降解,在体外分离的软骨细胞中也观察到类似的结果。时钟:Bmal1异二聚体与Pdgfa启动子上游的增强子结合以增强其转录。Pdgfa敲低通过灭活Pi3k/Akt和Erk1/2级联,逆转了Clock和Bmal1的保护作用。相比之下,Pdgfa过表达可减少软骨细胞损伤,而Pi3k/Akt抑制剂LY294002可消除这一作用。综上所述,本研究验证了CRD与OA的发展相关。通过激活Pdgfa转录和增强Pi3k/Akt和Erk1/2信号通路,恢复Clock和Bmal1水平可能有助于改善crd相关OA的软骨细胞损失。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


