Melatonin attenuates high-fat diet- and particulate matter-induced cardiac injury: involvement of mitochondrial quality and miR-221/222 expression
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
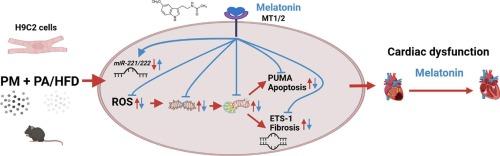
Previous studies have shown that exposure to hyperlipidemia or particulate matter (PM) individually affects the progression of cardiovascular disease (CVD), but the combined effects of these factors remain understudied. This study investigated whether combined treatment with a high-fat diet (HFD)/palmitate (PA) and PM exacerbates cardiomyocyte injury and proposed using the antioxidant melatonin. Furthermore, we explored the role of mitochondria and miR-221/222 in melatonin-mediated reduction of HFD- and PM-exacerbated cardiomyocyte injury. H9c2 cells were treated with or without 50 μM PA, 10 μg/mL PM, and 100 μM melatonin for 24 h. In the in vivo experiments, 8–12-week-old wild-type (WT) mice, miR-221/222 knockout (miR-221/222–/-) mice, and miR-221/222 overexpression (miR-221/222o/e) mice were treated with HFD for 4 weeks. PM was injected intratracheally at the end of the second and third weeks, and melatonin 20 mg/kg was administered orally daily starting at the end of the second week. Combined PA/HFD and PM induced mitochondrial ROS accumulation, subsequent mitochondrial fission, and excessive mitophagy in cardiomyocytes and cardiac tissues. This cascade increases cardiomyocyte apoptosis and fibrosis, leading to cardiac dysfunction. Melatonin treatment reduced mitochondrial ROS accumulation and improved HFD- and PM-induced cardiac dysfunction. Further exploration of the molecular mechanism highlighted that miR-221/222 upregulation is a downstream effect of melatonin, revealing a novel regulatory pathway for HFD- and PM-induced cardiac injury. This study showed that simultaneous exposure to HFD/PA and PM exacerbated cardiomyocyte apoptosis and fibrosis. These effects could be ameliorated by melatonin-mediated ROS scavenging, maintenance of mitochondrial function, and cardioprotection associated with miR-221/222.
褪黑激素减轻高脂肪饮食和颗粒物引起的心脏损伤:涉及线粒体质量和miR-221/222表达。
先前的研究表明,暴露于高脂血症或颗粒物(PM)单独影响心血管疾病(CVD)的进展,但这些因素的综合影响仍未得到充分研究。本研究探讨了高脂肪饮食(HFD)/棕榈酸酯(PA)和PM联合治疗是否会加重心肌细胞损伤,并建议使用抗氧化褪黑激素。此外,我们探讨了线粒体和miR-221/222在褪黑激素介导的HFD和pm加重心肌细胞损伤中的作用。分别用50 μM PA、10 μg/mL PM和100 μM褪黑素处理或不处理H9c2细胞24 h。在体内实验中,8-12周龄野生型(WT)小鼠、miR-221/222敲除(miR-221/222-/-)小鼠和miR-221/222过表达(mir -221/ 2220 /e)小鼠用HFD处理4 周。在第二周和第三周末气管内注射PM,从第二周末开始每日口服褪黑素20 mg/kg。PA/HFD联合PM诱导心肌细胞和心脏组织的线粒体ROS积累,随后的线粒体分裂和过度的线粒体自噬。这种级联反应增加心肌细胞凋亡和纤维化,导致心功能障碍。褪黑素治疗可减少线粒体ROS积累,改善HFD和pm诱导的心功能障碍。分子机制的进一步探索表明,miR-221/222上调是褪黑激素的下游效应,揭示了HFD和pm诱导的心脏损伤的新调控途径。本研究表明,同时暴露于HFD/PA和PM会加剧心肌细胞凋亡和纤维化。这些作用可以通过褪黑素介导的ROS清除、线粒体功能的维持以及与miR-221/222相关的心脏保护来改善。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


