Generation of a potent & selective series of IRAK4 inhibitors based on a structure based, hybridization approach
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
IRAK4 inhibitors are of high interest as treatment for not only inflammatory and autoimmune diseases, but also in the field of oncology. Despite extensive research in the IRAK4 area and progression of several inhibitors into clinical trials, no IRAK4 inhibitor has yet reached the market. In this article, we describe the development of a highly potent and selective IRAK4 lead compound 5e, starting from Astellas AS2444697 (1a). The work includes identification of the pyrazole-pyridine substituent in compound 1g, binding towards the gatekeeper region of IRAK4, followed by a structure-guided scaffold hybridization that led to 5a. Subsequent optimization of substituents of the indazole scaffold yielded 5e, which exhibits a 10-fold improvement in IRAK4 cell potency and higher off-target selectivity compared to AS244697 (1a).
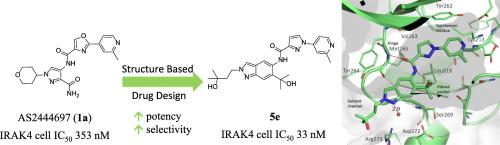
基于基于结构的杂交方法,产生有效和选择性的IRAK4抑制剂系列。
IRAK4抑制剂不仅作为炎症和自身免疫性疾病的治疗,而且在肿瘤学领域也受到高度关注。尽管在IRAK4领域进行了广泛的研究,并有几种抑制剂进入临床试验,但尚未有IRAK4抑制剂进入市场。在这篇文章中,我们描述了一种高效和选择性的IRAK4先导化合物5e的开发,从安斯泰来AS2444697 (1a)开始。这项工作包括鉴定化合物1g中的吡唑-吡啶取代基,结合到IRAK4的看门人区域,然后进行结构引导的支架杂交,得到5a。随后对吲哚唑支架的取代基进行优化,得到5e,与AS244697相比,其IRAK4细胞效力提高了10倍,脱靶选择性更高(1a)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


