Evolving roles for the androgen receptor and its protein interactome in castration-resistant prostate cancer
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The androgen receptor (AR) is a ligand-activated transcription factor that is a major driver of lethal prostate cancer (CaP) progression. Androgen deprivation therapy (ADT) that prevents the binding of androgens to AR has been the mainstay for the treatment of non-organ-confined CaP for more than 8 decades. Although ADT initially induces remissions, eventually resistance occurs while the majority of castration-resistant CaPs (CRPCs) continue to rely on AR’s action for growth. Sustained AR-dependence of CaP that recurs under ADT has historically been linked to AR’s transcriptional activity that controls expression of a distinct program of target genes that mediate aggressive behavior. Recently, less traditional transcriptional roles for AR, such as those impacting non-coding RNAs as well as transcription-independent roles that include AR-dependent splicing programs and translation control have been recognized to contribute to aggressive CaP features and treatment resistance. We reviewed and contrasted the contribution and relevance of these distinct functions for AR during CaP progression. We also considered the roles therein, both overlapping or mutually exclusive, for functionally diverse AR-interacting proteins that have been identified and to date have been mostly considered AR-associated transcriptional regulators. We discuss the potential implications of the involvement of AR interactors in multiple AR-dependent (non-)transcriptional cellular processes for alternative CaP treatment strategies that disrupt AR-coregulator interplay to inhibit AR-dependent transcription when AR ligand-deprivation has failed.
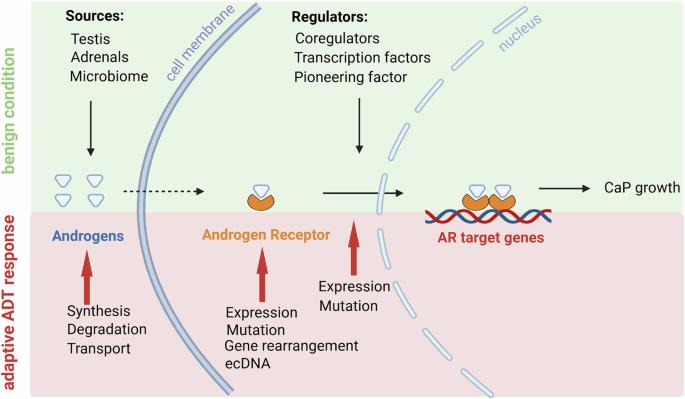
雄激素受体及其蛋白相互作用组在去势抵抗性前列腺癌中的进化作用。
雄激素受体(AR)是一种配体激活的转录因子,是致死性前列腺癌(CaP)进展的主要驱动因素。80多年来,雄激素剥夺疗法(ADT)阻止雄激素与AR结合一直是治疗非器官限制性CaP的主要方法。虽然ADT最初会引起缓解,但最终会发生耐药性,而大多数去势抗性cap (crpc)继续依赖AR的作用来生长。在ADT下反复出现的CaP的持续AR依赖性历史上与AR的转录活性有关,AR的转录活性控制介导攻击行为的不同靶基因程序的表达。最近,一些不太传统的AR转录作用,如那些影响非编码rna的转录作用,以及转录无关的作用,包括AR依赖的剪接程序和翻译控制,已被认为有助于侵略性CaP特征和治疗抗性。我们回顾并对比了这些不同功能在CaP进展过程中对AR的贡献和相关性。我们还考虑了其中的作用,无论是重叠的还是互斥的,对于已经确定的功能多样的ar相互作用蛋白,迄今为止主要被认为是ar相关的转录调节因子。我们讨论了AR相互作用物参与多个AR依赖(非)转录细胞过程的潜在影响,当AR配体剥夺失败时,替代的CaP治疗策略会破坏AR共调节物的相互作用以抑制AR依赖的转录。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


