Optimised formula of Fufang Biejia Ruangan tablets alleviates renal fibrosis by suppressing matrix-stiffness–induced fibroblast activation via inhibition of integrin αVβ1 binding
IF 5.4
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Ethnopharmacological relevance
Fufang Biejia Ruangan Tablets (FFBJ), a traditional Chinese medicine, has long been used in the management of fibrosis, demonstrating notable efficacy and safety. However, the bioactive components of FFBJ and their mechanisms of action remain poorly understood.
Aim of the study
To identify the core bioactive components of FFBJ and elucidate their mechanisms of action against renal fibrosis.
Materials and methods
The constituents of FFBJ were characterised using high-performance liquid chromatography–High-resolution mass spectrometry (HPLC-HRMS). A multi-tiered strategy integrating pharmacokinetic evaluation, ADMET predictions, molecular docking, and efficacy assessments (MTT/Sirius-Red assays) identified five core bioactive compounds. The synergistic ratios of these compounds were optimised using the Box–Behnken design. In vitro, TGF-β1–stimulated NRK49F cells were treated with FFBJ-COF, a compound composed optimised formula from FFBJ, at low (half-medium dose), medium (30 μM taxifolin, 7.732 μM turtle-shell heptapeptide [OC1], 13.623 μM quercetin, 13 μM curcumenol, and 3.3 μM hederagenin), or high (double-medium dose) concentrations. In vivo, mice with unilateral ureteral obstruction (UUO) were treated with FFBJ-COF via gavage at low (half-medium dose), medium (64.85 mg/kg/d taxifolin, 30.30 mg/kg/d turtle-shell heptapeptide [OC1], 29.25 mg/kg/d quercetin, 21.83 mg/kg/d curcumenol, and 10.07 mg/kg/d hederagenin), or high (1.5-fold medium dose) levels. Polyacrylamide hydrogels (1–20 kPa) were employed to mimic fibrotic-matrix stiffness. Co-immunoprecipitation and western blotting were used to investigate the regulation of integrin αVβ1 binding and the downstream FAK/RhoA/MRTF-A signalling.
Results
HPLC-HRMS analysis revealed 76 components. FFBJ-COF, comprising curcumenol, hederagenin, taxifolin, quercetin, and OC1, was formulated based on efficacy screening and ratio optimization. FFBJ-COF treatment attenuated renal injury and fibrosis by reducing fibroblast activation and extracellular matrix (ECM) deposition in both in vivo and in vitro model. Mechanistic studies showed that ECM stiffness activated fibroblasts, which was inhibited by FFBJ-COF. Co-immunoprecipitation demonstrated that matrix stiffness enhanced the binding of integrin αVβ1, whereas FFBJ-COF suppressed this interaction and inhibited the downstream FAK/RhoA/MRTF-A signalling pathway.
Conclusion
FFBJ-COF was identified as the core bioactive fraction of FFBJ. FFBJ-COF exerts anti-fibrotic effects by inhibiting integrin αVβ1 binding, thereby suppressing the FAK/RhoA/MRTF-A signalling and disrupting the positive feedback loop between ECM stiffness and fibrosis. These findings highlight the potential of FFBJ as a novel therapeutic agent for renal fibrosis.
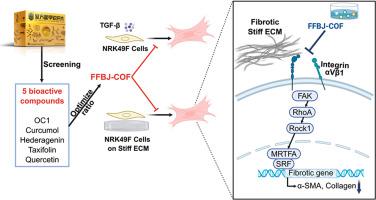
复方别甲软肝片优化配方通过抑制整合素αVβ1结合抑制基质刚度诱导的成纤维细胞活化减轻肾纤维化。
民族药理学相关性:复方别甲软肝片(FFBJ)是一种中药,长期用于治疗纤维化,疗效和安全性显著。然而,FFBJ的生物活性成分及其作用机制仍然知之甚少。研究目的:鉴定白肾参的核心生物活性成分,并阐明其抗肾纤维化的作用机制。材料与方法:采用高效液相色谱-高分辨率质谱法(HPLC-HRMS)对其成分进行表征。结合药代动力学评估、ADMET预测、分子对接和疗效评估(MTT/Sirius-Red测定)的多层策略确定了五种核心生物活性化合物。使用Box-Behnken设计优化这些化合物的协同比例。体外,以TGF-β1刺激的NRK49F细胞为实验对象,分别以低浓度(半中剂量)、中浓度(30 μM杉木素、7.732 μM甲壳七肽[OC1]、13.623 μM槲皮素、13 μM姜黄烯醇、3.3 μM hederagenin)和高浓度(双中剂量)处理NRK49F细胞。在体内,对单侧输尿管梗阻(UUO)小鼠进行低(半中剂量)、中(64.85 mg/kg/d taxifolin、30.30 mg/kg/d甲壳七肽[OC1]、29.25 mg/kg/d槲皮素、21.83 mg/kg/d姜黄酚、10.07 mg/kg/d hederagenin)、高(1.5倍中剂量)灌胃治疗。采用聚丙烯酰胺水凝胶(1-20 kPa)模拟纤维基质刚度。采用免疫共沉淀法和免疫印迹法研究整合素αVβ1结合及下游FAK/RhoA/MRTF-A信号的调控。结果:HPLC-HRMS分析共检出76种成分。FFBJ-COF由姜黄酚、hederagenin、taxifolin、槲皮素和OC1组成。在体内和体外模型中,FFBJ-COF通过降低成纤维细胞活化和细胞外基质(ECM)沉积来减轻肾损伤和纤维化。机制研究表明,ECM硬度激活成纤维细胞,而FFBJ-COF抑制了成纤维细胞。共免疫沉淀表明,基质硬度增强了整合素αVβ1的结合,而FFBJ-COF抑制了这种相互作用,并抑制了下游FAK/RhoA/MRTF-A信号通路。结论:FFBJ- cof为FFBJ的核心活性部位。FFBJ-COF通过抑制整合素αVβ1结合发挥抗纤维化作用,从而抑制FAK/RhoA/MRTF-A信号通路,破坏ECM僵硬度与纤维化之间的正反馈回路。这些发现突出了FFBJ作为一种新型肾纤维化治疗剂的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of ethnopharmacology
医学-全科医学与补充医学
CiteScore
10.30
自引率
5.60%
发文量
967
审稿时长
77 days
期刊介绍:
The Journal of Ethnopharmacology is dedicated to the exchange of information and understandings about people''s use of plants, fungi, animals, microorganisms and minerals and their biological and pharmacological effects based on the principles established through international conventions. Early people confronted with illness and disease, discovered a wealth of useful therapeutic agents in the plant and animal kingdoms. The empirical knowledge of these medicinal substances and their toxic potential was passed on by oral tradition and sometimes recorded in herbals and other texts on materia medica. Many valuable drugs of today (e.g., atropine, ephedrine, tubocurarine, digoxin, reserpine) came into use through the study of indigenous remedies. Chemists continue to use plant-derived drugs (e.g., morphine, taxol, physostigmine, quinidine, emetine) as prototypes in their attempts to develop more effective and less toxic medicinals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


