Yu-Ping-Feng nasal drops relieve allergic rhinitis via TRPV1/Ca2+/NFAT pathway
IF 5.4
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Ethnopharmacological relevance
Allergic rhinitis (AR) is a chronic inflammatory disorder of the nasal mucosa mediated by immunoglobulin E (IgE). Yu-Ping-Feng nasal drops (YPFND), a compound derived from traditional Chinese medicine, is extensively utilized in the management of respiratory ailments, including AR. Nonetheless, the precise biological pathways through which YPFND influences AR are yet to be fully elucidated.
Purpose
We aim to investigate the bioactivity of YPFND against AR and its therapeutic potential molecular mechanism.
Methods
We conducted a 14-day randomized controlled trial involving 60 AR patients assigned to control group (10 % YPFND solution, Con group), mometasone furoate group (MFN group), and YPFND group, with nasal symptom outcomes assessed post-treatment. In parallel, an ovalbumin (OVA)- developed AR rat model was established to investigate the effect of YPFND on nasal mucosal inflammation. Nasal tissues and serum were analyzed by ELISA, Western blot, quantitative PCR (qPCR), histology, immunofluorescence and flow cytometry. Chemical constituents of YPFND were identified via LC-MS/MS. Serum proteomics in control and OVA-induced AR rats was analyzed by nano-HPLC–MS/MS with DIA-NN identification and MaxLFQ quantification. Molecular docking and molecular dynamics (MD) simulations were performed to predict the binding affinity of active compounds to the transient receptor potential vanilloid 1 (TRPV1) protein. In vitro, human nasal epithelial cells (HNEpC) were stimulated with interleukin-17 A (IL-17 A) to induce inflammation. The effects of YPFND on TRPV1-mediated Ca2+ influx and nuclear factor of activated T cells (NFAT) activation were assessed, and TRPV1 specificity was confirmed using the antagonist SB-366791.
Results
LC-MS/MS analysis identified 984 compounds, among which molecular docking revealed nine candidates exhibiting strong binding affinity to TRPV1. Subsequently, MD simulations confirmed the stability of the 5-O-methylvisammioside-TRPV1 complex. Quantitative proteomics quantified 9978 proteins and identified 527 significantly altered proteins (259 upregulated, 268 downregulated) between OVA-induced AR and control rats, enriched in pathways including inflammatory mediator regulation of TRP channels. YPFND significantly alleviated clinical and experimental AR symptoms. In HNEpC, IL-17 A stimulated the expression of TRPV1 and facilitated Ca2+ influx. YPFND inhibited the IL-17 A-provoked NFAT expression via the TRPV1/Ca2+ signaling pathway. In addition, YPFND ameliorated airway inflammation by inhibiting the TRPV1/NFAT pathway in AR rats and down-regulated the expression of retinoic acid receptor-related orphan receptor gamma t (RORγ-t), further regulating the balance between T helper 17 (Th17) cells and regulatory T (Treg) cells.
Conclusion
Our research elucidates a novel IL-17A/TRPV1/Ca2+/NFAT signaling cascade as a critical mediator of Th17/Treg disequilibrium in AR. Moreover, it demonstrates that YPFND exerts anti-inflammatory effects by attenuating TRPV1-dependent Ca2+ influx and its downstream NFAT activation.
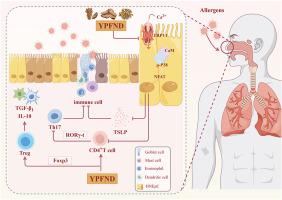
玉屏风滴鼻液通过TRPV1/Ca2+/NFAT通路缓解变应性鼻炎。
民族药理学相关性:变应性鼻炎(AR)是一种由免疫球蛋白E (IgE)介导的鼻黏膜慢性炎症性疾病。玉平风滴鼻液(YPFND)是一种从中药中提取的化合物,广泛用于治疗呼吸系统疾病,包括AR。然而,YPFND影响AR的确切生物学途径尚未完全阐明。目的:研究YPFND抗AR的生物活性及其潜在的分子机制。方法:我们对60例AR患者进行了为期14天的随机对照试验,将其分为对照组(10% YPFND溶液,Con)、糠酸莫米松组(MFN组)和YPFND组,并评估治疗后的鼻症状结局。同时,建立卵清蛋白(OVA)诱导的AR大鼠模型,探讨YPFND对鼻黏膜炎症的影响。采用ELISA、western blot、定量PCR (qPCR)、组织学、免疫荧光和流式细胞术分析鼻部组织和血清。通过LC-MS/MS对其化学成分进行鉴定。采用纳米高效液相色谱-质谱(hplc -MS/MS)技术对对照组和ova诱导的AR大鼠血清蛋白质组学进行分析,并结合DIA-NN鉴定和MaxLFQ定量。通过分子对接和分子动力学(MD)模拟来预测活性化合物与瞬时受体电位香草蛋白1 (TRPV1)蛋白的结合亲和力。体外用白细胞介素- 17a (IL-17A)刺激人鼻上皮细胞(HNEpC)诱导炎症反应。评估了YPFND对TRPV1介导的Ca2+内流和活化T细胞核因子(NFAT)活化的影响,并使用拮抗剂SB-366791确认了TRPV1的特异性。结果:LC-MS/MS分析鉴定出984个化合物,其中分子对接发现9个候选化合物与TRPV1具有较强的结合亲和力。随后,MD模拟证实了5- o -甲基维沙米苷- trpv1复合物的稳定性。定量蛋白质组学测定了9978个蛋白,鉴定出527个蛋白在ova诱导的AR和对照大鼠之间发生了显著改变(259个上调,268个下调),这些蛋白在炎症介质调节TRP通道等途径中富集。YPFND可显著缓解AR临床和实验症状。在HNEpC中,IL-17A刺激TRPV1的表达,促进Ca2+内流。YPFND通过TRPV1/Ca2+信号通路抑制IL-17A诱导的NFAT表达。此外,YPFND通过抑制AR大鼠TRPV1/NFAT通路改善气道炎症,下调RORγ-t表达,进一步调节Th17/Treg平衡。结论:我们的研究阐明了IL-17A/TRPV1/Ca2+/NFAT信号级联是AR中Th17/Treg失衡的关键介质。此外,研究表明YPFND通过减弱TRPV1依赖的Ca2+内流及其下游NFAT激活来发挥抗炎作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of ethnopharmacology
医学-全科医学与补充医学
CiteScore
10.30
自引率
5.60%
发文量
967
审稿时长
77 days
期刊介绍:
The Journal of Ethnopharmacology is dedicated to the exchange of information and understandings about people''s use of plants, fungi, animals, microorganisms and minerals and their biological and pharmacological effects based on the principles established through international conventions. Early people confronted with illness and disease, discovered a wealth of useful therapeutic agents in the plant and animal kingdoms. The empirical knowledge of these medicinal substances and their toxic potential was passed on by oral tradition and sometimes recorded in herbals and other texts on materia medica. Many valuable drugs of today (e.g., atropine, ephedrine, tubocurarine, digoxin, reserpine) came into use through the study of indigenous remedies. Chemists continue to use plant-derived drugs (e.g., morphine, taxol, physostigmine, quinidine, emetine) as prototypes in their attempts to develop more effective and less toxic medicinals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


