Icariside II ameliorates slow transit constipation by inhibiting macrophage polarization and suppressing the cGAS-STING pathway
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Background
Slow transit constipation (STC) is a functional disorder characterized by slowed colonic peristalsis and delayed emptying. Its pathogenesis involves enteric nervous system damage and immune dysregulation, among other factors. Icariside II (ICS II) is a natural flavonoid glycoside from Herba Epimedii and is known for its anti-inflammatory, antioxidant, and neuroprotective effects. However, the effect of ICS II on STC and the underlying mechanisms remains unclear.
Methods
In this study, the effects of ICS II on STC were assessed in a loperamide-induced STC animal model. Drug efficacy was evaluated by observing the general phenotype using hematoxylin and eosin staining, immunofluorescence, Western blotting, and flow cytometry. Additionally, the roles of intestinal macrophages and the cyclic guanosine monophosphate-adenosine synthase/stimulator of interferon genes (cGAS-STING) signaling pathway in STC were studied using clodronate liposomes and STING inhibitor.
Results
ICS II treatment significantly increased fecal count, fecal moisture content, and intestinal propulsion rate, shortened first dark fecal defecation time, and improved colonic histopathology in the STC animal model. Notably, ICS II reduced intestinal M1-type macrophage proportion, downregulated proteins in the cGAS-STING signaling pathway, and lowered the release of inflammatory factors interleukin (IL)-1β, IL-6, and tumor necrosis factor-α. ICS II also decreased intestinal neuronal damage and increased nerve fiber density in STC disease, demonstrating its anti-inflammatory and neuroprotective effects.
Conclusion
This study provides evidence that ICS II exerts significant anti-inflammatory and neuroprotective effects. This is achieved by inhibiting the cGAS-STING pathway and suppressing macrophage M1 polarization, suggesting its potential as a therapeutic agent for STC.
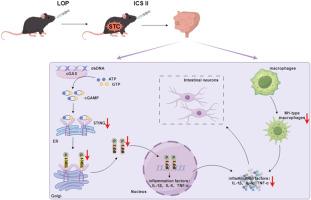
Icariside II通过抑制巨噬细胞极化和抑制cGAS-STING通路改善慢传输型便秘。
背景:慢传输型便秘(STC)是一种以结肠蠕动减慢和排空延迟为特征的功能性疾病。其发病机制涉及肠神经系统损伤和免疫失调等因素。Icariside II (ICS II)是从淫羊藿中提取的一种天然类黄酮苷,具有抗炎、抗氧化和神经保护作用。然而,ICS II对STC的影响及其潜在机制尚不清楚。方法:采用洛哌丁胺致STC动物模型,观察ICSⅱ对STC的影响。采用苏木精和伊红染色、免疫荧光、Western blotting和流式细胞术观察总表型,评价药物疗效。此外,使用氯膦酸脂质体和STING抑制剂研究肠巨噬细胞和环鸟苷单磷酸腺苷合成酶/干扰素基因刺激因子(cGAS-STING)信号通路在STC中的作用。结果:ICS II处理显著提高了STC动物模型的粪便计数、粪便水分含量和肠道推进率,缩短了首次暗粪排便时间,改善了结肠组织病理学。值得注意的是,ICS II降低了肠道m1型巨噬细胞比例,下调了cGAS-STING信号通路中的蛋白,降低了炎症因子白细胞介素(IL)-1β、IL-6和肿瘤坏死因子-α的释放。ICS II还可减少STC病的肠神经元损伤,增加神经纤维密度,显示其抗炎和神经保护作用。结论:本研究证明了ICS II具有明显的抗炎和神经保护作用。这是通过抑制cGAS-STING途径和抑制巨噬细胞M1极化来实现的,表明其作为STC治疗剂的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


