Lipid-lowering effect of evolocumab in hypertriglyceridemia-induced acute pancreatitis
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Background
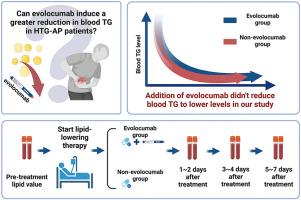
The primary goal of treatment for hypertriglyceridemia-induced acute pancreatitis (HTG-AP) is to rapidly reduce blood triglyceride (TG) level to below 5.65 mmol/L. Evolocumab with a significant lipid-lowering effect, has been widely used for cardiovascular diseases. However, it has been rarely explored in HTG-AP.
Methods
HTG-AP patients, who were admitted between January 2022 and February 2024, were retrospectively reviewed. They were further divided according to the use of evolocumab. Blood TG levels before treatment were compared with those after 1–2 days, 3–4 days, and 5–7 days of treatment.
Results
Overall, 62 patients were included, of whom 30 (48.4 %) were in the evolocumab group and 32 (51.6 %) were in the non-evolocumab group. The median pre-treatment TG levels were not significantly different between them (22.17 mmol/L vs 16.94 mmol/L, P = 0.108). Blood TG levels after 1–2 days (P = 0.016) and 5–7 days (P = 0.007) were significantly higher in the evolocumab group than the non-evolocumab group. The proportion of TG < 5.65 mmol/L after 1–2 days (6/24 vs 11/23, P = 0.104) and 3–4 days (10/19 vs 14/20, P = 0.265) was not significantly different between them, but that after 5–7 days (9/15 vs 19/20, P = 0.033) was significantly lower in the evolocumab group than the non-evolocumab group.
Conclusion
The addition of evolocumab to conventional lipid-lowering therapy did not produce any additional benefit in the reduction of blood TG levels in HTG-AP patients.
evolocumab在高甘油三酯血症诱导的急性胰腺炎中的降脂作用。
背景:治疗高甘油三酯血症引起的急性胰腺炎(HTG-AP)的主要目标是迅速降低血液甘油三酯(TG)水平至5.65 mmol/L以下。Evolocumab具有显著的降脂作用,已广泛用于心血管疾病。然而,在HTG-AP中很少对其进行探索。方法:回顾性分析2022年1月至2024年2月住院的HTG-AP患者。他们根据evolocumab的使用情况进一步划分。比较治疗前和治疗后1 ~ 2天、3 ~ 4天和5 ~ 7天的血TG水平。结果:总体纳入62例患者,其中30例(48.4%)属于evolocumab组,32例(51.6%)属于非evolocumab组。治疗前TG水平中位数在两组间无显著差异(22.17 mmol/L vs 16.94 mmol/L, P=0.108)。evolocumab组在1 ~ 2天(P=0.016)和5 ~ 7天(P=0.007)后的血TG水平显著高于非evolocumab组。结论:在常规降脂治疗中加入evolocumab对降低HTG-AP患者的血TG水平没有产生任何额外的益处。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


