Restoring nitric oxide/Peroxynitrite equilibrium in impaired human neural progenitor cells: Nanomedical approaches and their potential impact on neurodegenerative disease treatment
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
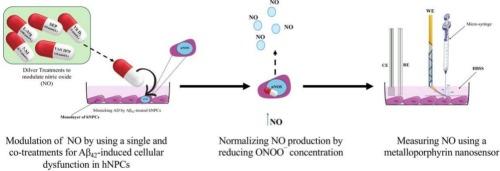
Nitric oxide (NO), an essential inorganic signaling molecule, involved in many physiological processes and has promising therapeutic potential Its oxidation product, peroxynitrite (ONOO¯), is cytotoxic, and elevated ONOO¯ levels induce nitroxidative stress, a factor implicated in the pathogenesis of neurodegenerative diseases such as Alzheimer's disease (AD). Through pharmacological modulation of NO and ONOO¯ levels in human neural progenitor cell (hNPCs), this study explores the potential pharmaceutical interventions targeting the NO and the neuronal nitric oxide synthase (nNOS) pathway, (NO/nNOS), to prevent or reduce AD progression by restoring the [NO]/[ONOO¯] balance. To achieve this, metalloporphyrin nanosensors have been effectively employed for real-time, in-situ measurement of NO and ONOO¯ concentrations (200–300 nm diameter) were applied and precisely positioned 4–5 ± 1 μm from hNPCs membranes, enabling precise investigation of the [NO]/[ONOO¯] ratio. The [NO]/[ONOO¯] ratio emerged as a critical biomarker for the evaluation of nNOS coupling/uncoupling to the hNPC functioning/dysfunction. In healthy cells, this ratio was around 0.25 ± 0.005, While dysfunctional hNPCs treated to amyloid beta 42 (Aβ42)—a hallmark of AD—caused a dramatic 94 % drop, signaling severe cellular dysfunction. Based on these findings, potential pharmacological interventions have been proposed to prevent or reduce AD progression by restoring the [NO]/[ONOO¯] balance. Notably, a co-treatment of sepiapterin (SEP), a cofactor precursor for NO synthesis, with VAS 2870 (an NADPH oxidase inhibitor) partially restored the ratio to 0.1, indicating improved nNOS function.
在受损的人类神经祖细胞中恢复一氧化氮/过氧亚硝酸盐平衡:纳米医学方法及其对神经退行性疾病治疗的潜在影响
一氧化氮(NO)是一种重要的无机信号分子,参与了许多生理过程,并具有良好的治疗潜力。其氧化产物过氧亚硝酸盐(ONOO¯)具有细胞毒性,ONOO¯水平升高会引起氮氧化应激,这是神经退行性疾病(如阿尔茨海默病(AD))发病的一个因素。通过对人神经祖细胞(hNPCs)中NO和ONOO¯水平的药理调节,本研究探索了针对NO和神经元一氧化氮合酶(nNOS)通路(NO/nNOS)的潜在药物干预,通过恢复[NO]/[ONOO¯]平衡来预防或减少AD的进展。为了实现这一目标,金属卟啉纳米传感器被有效地用于实时、原位测量NO和ONOO¯浓度(200-300 nm直径),并精确定位在离hNPCs膜4-5±1 μm处,从而能够精确地研究[NO]/[ONOO¯]比率。[NO]/[ONOO¯]比值成为评估nNOS偶联/解耦与hNPC功能/功能障碍的关键生物标志物。在健康细胞中,这一比例约为0.25±0.005,而功能失调的hNPCs经淀粉样蛋白β42 (a - β42)处理后,这一比例急剧下降94%,表明严重的细胞功能障碍。基于这些发现,人们提出了通过恢复[NO]/[ONOO¯]平衡来预防或减少AD进展的潜在药物干预措施。值得注意的是,将一氧化氮合成的辅助因子前体sepapterin (SEP)与一种NADPH氧化酶抑制剂VAS 2870共同处理后,该比值部分恢复至0.1,表明nNOS功能得到改善。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


