Identification and mechanism of hepatoprotective saponins and endogenous metabolites in the sweet variant of Gynostemma pentaphyllum
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Gynostemma pentaphyllum (Thunb.) Makino has been traditionally utilized as medicinal herb and function food in traditional Chinese medicine for treating chronic hepatic disorders. Sweet variants of G. pentaphyllum collected from different regions of China are cultivated as the primary resources for the preparation of curative products. We and other groups identified a series of dammarane-type triterpenoids enriched in the plant, such as gypenosides LVI and XLVI, as the bioactive components responsible for the hepatoprotective effect. However, their therapeutic potential may be constrained by low oral bioavailability and complex metabolic pathways. This study applied an in vitro analysis of gut microbiome-based metabolism to determine the major pathway of the gypenosides. Structure-activity relationships, pharmacokinetic studies, and pharmacodynamic results revealed that the removal of the C-3 saccharide chain is not only the main metabolic pathway in the intestinal tract but also generates the active hepatoprotective ingredient with higher bioavailability and potency than the proteotype. Notably, the investigation of underlying the mechanism demonstrated that the compounds inhibited the activation of hepatic stellate cells via the AMPK/P300/Smad3 signaling pathway. Collectively, these findings demonstrate that in vivo metabolism is critical for unlocking the therapeutic potential of orally administered sweet variant G. pentaphyllum, as this metabolic process releases active ingredients that overcome the bioavailability limitations of the parent gypenoside.
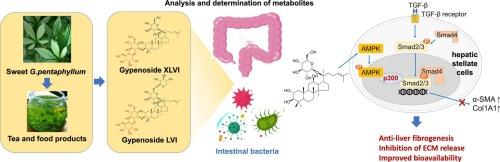
甜型绞股蓝保肝皂苷及其内源代谢产物的鉴定与机制研究。
绞股蓝(绞股蓝)牧野历来被用作中药和功能食品,用于治疗慢性肝病。来自中国不同地区的甜型五味子是制备治疗产品的主要资源。我们和其他研究小组鉴定了一系列富含达玛烷型三萜的植物,如绞股皂苷LVI和XLVI,作为负责保护肝脏作用的生物活性成分。然而,它们的治疗潜力可能受到口服生物利用度低和代谢途径复杂的限制。本研究应用体外肠道微生物代谢分析来确定绞股蓝皂苷的主要代谢途径。构效关系、药代动力学研究和药效学结果表明,C-3糖链的去除不仅是肠道内的主要代谢途径,而且还能产生比蛋白型具有更高生物利用度和效力的活性保肝成分。值得注意的是,对潜在机制的研究表明,这些化合物通过AMPK/P300/Smad3信号通路抑制肝星状细胞的激活。总的来说,这些发现表明,体内代谢对于释放口服甜变体五味子的治疗潜力至关重要,因为这种代谢过程释放的活性成分克服了母体绞股蓝苷的生物利用度限制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


