Visible light-promoted Pd-catalyzed regioselective 1,4-dicarbofunctionalization of 1,3-butadiene with unactivated bromides and malononitriles
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
As a versatile synthetic transformation, the dicarbofunctionalization of 1,3-butadiene enables the rapid assembly of structurally diverse allylic scaffolds. Herein, the selective 1,4-dicarbofunctionalization of 1,3-butadiene with organobromides (alkyl and aryl bromides) and malononitrile nucleophiles has been developed using a photoinduced Pd-catalyzed three-component coupling strategy. This process relies on photoexcited Pd(0) activation of both substrates through a single-electron transfer (SET) process, accommodating alkyl and aryl radicals of contrasting electronic character through precisely optimized reaction conditions. Under mild conditions, the protocol furnishes 74 functionalized malononitrile derivatives in high yields (up to 88%) and excellent regioselectivity (>20 : 1 rr), highlighting its broad scope and efficiency.
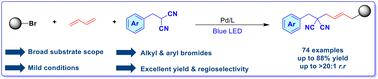
可见光促进pd催化1,3-丁二烯与非活化溴和丙二腈的区域选择性1,4-二碳功能化
作为一种多用途的合成转化,1,3-丁二烯的二碳功能化使得结构多样的烯丙基支架的快速组装成为可能。本文利用光诱导pd催化的三组分偶联策略,研究了1,3-丁二烯与有机溴化物(烷基溴和芳基溴)和丙二腈亲核试剂的选择性1,4-二碳功能化反应。该工艺依赖于光激发Pd(0)通过单电子转移(SET)过程激活两种底物,通过精确优化的反应条件容纳具有不同电子特征的烷基和芳基自由基。在温和的条件下,该方案以高收率(高达88%)和优异的区域选择性(> 20:1 rr)提供74个功能化丙二腈衍生物,突出了其广泛的适用范围和效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


