Noninvasive in vivo discrimination between mitochondrial ROS and global ROS production in solid tumors using EPR spectroscopy
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Because the precise site of ROS production plays a key role in cellular redox signaling and its (patho)physiological consequences, it is crucial to develop tools that enable site-specific detection of ROS in complex systems, including in vivo. Here, we propose the use of Electron Paramagnetic Resonance (EPR) and dual nitroxide sensors composed of mitoTEMPO and carbamoyl-proxyl (3CP) to probe ROS production in the mitochondrial and intracellular/extracellular compartments, respectively. For the proof-of-concept, the decay rates of the nitroxides were measured in 4T1 breast tumor models, both in vitro and in vivo, using 9 GHz and 1 GHz spectrometers, respectively. To modulate the level of ROS either in the cytosol or in the mitochondria, cells and mice were treated with either the glutathione synthesis inhibitor l-Buthionine Sulfoximine (L-BSO) or Antimycin A, an inhibitor of the complex III of the mitochondrial electron transport chain, or their appropriate controls. In mice, an increase in relative decay rate was observed for 3CP, but not for mitoTEMPO, 1 and 2 days after starting L-BSO treatment, while the opposite result was obtained after Antimycin A treatment. These observations were consistent with results obtained on cells in vitro. Ex-vivo analyses of tumors, with or without ferricyanide that converts hydroxylamines back to nitroxides, revealed non-significant changes in the total amount of nitroxide + hydroxylamine, suggesting that the blood wash-out did not play a role in the decay of the nitroxide signal. In addition, the use of genetically engineered 4T1 cells that overexpress the mitochondrial isoform superoxide dismutase 2 (SOD2) allowed the assessment of the contribution of superoxide production to EPR signal decay. Overall, this study identifies a new protocol to noninvasively discriminate the site of ROS production in vivo.
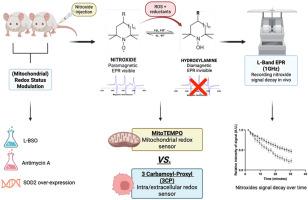
利用EPR光谱对实体肿瘤中线粒体ROS和整体ROS产生的无创体内鉴别。
由于ROS产生的精确位点在细胞氧化还原信号及其(病理)生理后果中起着关键作用,因此开发能够在复杂系统(包括体内)中对ROS进行位点特异性检测的工具至关重要。在这里,我们提出使用电子顺磁共振(EPR)和由mitoTEMPO和氨基酰基proxyl (3CP)组成的双氮氧化物传感器分别探测线粒体和细胞内/细胞外区室中的ROS产生。为了验证这一概念,在4T1乳腺肿瘤模型中,分别使用9 GHz和1 GHz光谱仪测量了氮氧化物的衰变率,包括体外和体内。为了调节细胞质或线粒体中的ROS水平,细胞和小鼠被谷胱甘肽合成抑制剂l-丁硫氨酸亚砜(L-BSO)或抗霉素A(线粒体电子传递链复合物III的抑制剂)或其适当的对照物处理。在小鼠中,开始L-BSO治疗后1和2天,3CP的相对衰减率增加,而mitoTEMPO没有增加,而抗霉素A治疗后的结果相反。这些观察结果与在体外细胞上获得的结果一致。对肿瘤的离体分析显示,无论有无铁氰化物将羟胺转化为氮氧化物,氮氧化物+羟胺的总量都没有显著变化,这表明血液冲洗在氮氧化物信号的衰减中没有发挥作用。此外,使用过表达线粒体异构体超氧化物歧化酶2 (SOD2)的基因工程4T1细胞,可以评估超氧化物产生对EPR信号衰减的贡献。总的来说,本研究确定了一种新的无创识别体内ROS产生部位的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


