Redox-dependent liver gluconeogenesis impacts different intensity exercise in mice
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Hepatic gluconeogenesis produces glucose from various substrates to meet energy demands. However, how these substrates are preferentially used under different conditions remains unclear. Here, we show that preferential supplies of lactate and glycerol modulate hepatic gluconeogenesis, thereby impacting high-intensity and low-intensity exercise capacities, respectively. We find that liver-specific knockout of phosphoenolpyruvate carboxykinase 1 (L-Pck1KO), which blocks gluconeogenesis from lactate, decreases high-intensity exercise capacity but increases low-intensity exercise capacity by enhancing gluconeogenesis from glycerol. Conversely, liver-specific knockout of glycerol kinase (L-GykKO), which inhibits glycerol-derived gluconeogenesis, induces the opposite effects by enhancing gluconeogenesis from lactate. Given that these compensatory steps depend on NAD+-mediated oxidation in the cytosol, we hepatically expressed NADH oxidase from Lactobacillus brevis (LbNOX) to decrease the cytosolic [NADH]/[NAD+] ratio. We find that hepatic LbNOX expression enhances gluconeogenesis from both redox-dependent substrates and increases exercise capacities at both intensities. Importantly, LbNOX-induced enhancement of high-intensity and low-intensity exercise capacities is abolished in L-Pck1KO and L-GykKO mice, respectively. Therefore, supplies of gluconeogenic substrates and cytosolic redox states, rather than altered enzyme expressions, modulate hepatic gluconeogenesis and exercise capacity at different intensities. Globally, this study shows that regulating hepatic gluconeogenesis through cytosolic redox states is a potent strategy for increasing exercise performance. This study shows that the preferential use of gluconeogenic pathways in the liver depends on exercise load.
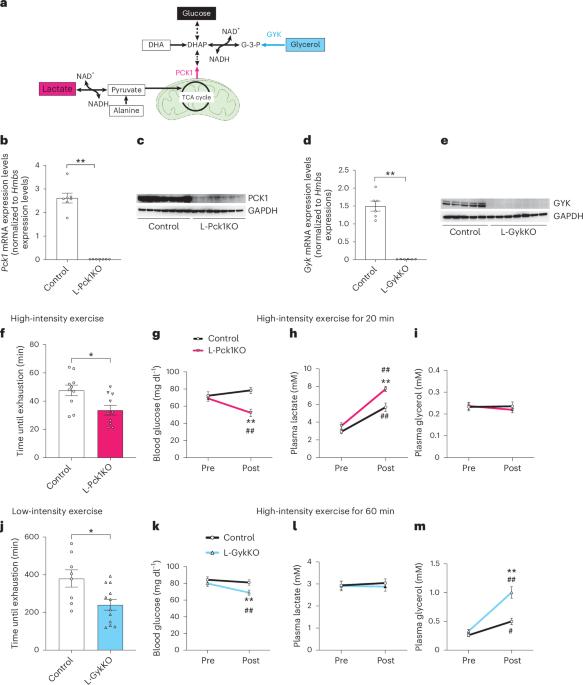
氧化还原酶依赖性肝糖异生对小鼠不同强度运动的影响。
肝脏糖异生从各种底物中产生葡萄糖以满足能量需求。然而,这些底物在不同条件下如何优先使用仍不清楚。在这里,我们表明乳酸和甘油的优先供应调节肝脏糖异生,从而分别影响高强度和低强度运动能力。我们发现肝脏特异性敲除磷酸烯醇丙酮酸羧激酶1 (L-Pck1KO),它可以阻断乳酸的糖异生,降低高强度运动能力,但通过增强甘油的糖异生来增加低强度运动能力。相反,肝脏特异性敲除甘油激酶(L-GykKO),抑制甘油衍生的糖异生,通过增强乳酸糖异生诱导相反的效果。考虑到这些补偿步骤依赖于细胞质中NAD+介导的氧化,我们在肝脏中表达了来自短乳杆菌(LbNOX)的NADH氧化酶,以降低细胞质[NADH]/[NAD+]的比例。我们发现肝脏LbNOX的表达增强了氧化还原依赖底物的糖异生,并增加了两种强度下的运动能力。重要的是,lbnox诱导的高强度和低强度运动能力的增强在L-Pck1KO和L-GykKO小鼠中分别被消除。因此,糖异生底物的供应和胞质氧化还原状态,而不是改变酶的表达,在不同强度下调节肝脏糖异生和运动能力。在全球范围内,这项研究表明,通过胞质氧化还原状态调节肝脏糖异生是提高运动表现的有效策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


