Disruption of mitochondria-associated membranes contributes to the dysregulation of insulin secretion in undernutrition, obesity, and double burden of malnutrition
IF 11.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Aims/hypothesis
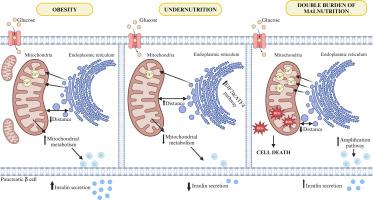
Nutritional disorders directly affect the endocrine pancreas, increasing the susceptibility to type 2 diabetes mellitus. However, the molecular mechanisms underlying these alterations remain unknown. This study aims to characterize the role of endoplasmic reticulum (ER)-mitochondria contact sites, known as mitochondrial-associated membranes (MAMs), in insulin secretion dysfunctions associated with undernutrition, obesity, and the double burden of malnutrition (DBM).
Methods
Rat pancreatic INS-1E β-cells were cultured in a medium without amino acids supplemented with 1 × (control) or 0.25 × (amino acid restriction) of an amino acid solution for 48 h, and then cells were exposed to a fatty acid mix for 48 h. Male C57BL/6 mice were fed a normoprotein diet (14 % protein) or protein-restricted diet (6 % protein) for 6 weeks and subsequently a high-fat diet (35 % kcal) for 12 weeks. ER-mitochondria interactions were evaluated by in situ proximity ligation assay and transmission electronic microscopy.
Results
Our findings indicate that protein restriction reduces ER-mitochondria contacts in pancreatic beta-cells, leading to decreased mitochondrial metabolism and glucose-stimulated insulin secretion (GSIS). In contrast, obesity increases ER-mitochondria contact points, mitochondrial metabolism, and GSIS in pancreatic beta-cells, without alterations in viability. DBM results in a significant increase in ER-mitochondria contacts, elevated mitochondrial calcium levels, increased production of reactive oxygen species, and cell death, collectively contributing to impaired GSIS response in the context of obesity.
Conclusions/interpretation
These data indicates that MAMs play a crucial role in GSIS during nutritional disorders such as undernutrition, obesity, and DBM. Importantly, changes in MAMs precede GSIS impairment, therefore targeting these interactions might prevent further disruption in beta-cell function.
线粒体相关膜的破坏有助于营养不良、肥胖和营养不良双重负担中胰岛素分泌的失调。
目的/假设:营养失调直接影响胰腺内分泌,增加2型糖尿病的易感性。然而,这些变化背后的分子机制尚不清楚。本研究旨在描述内质网(ER)-线粒体接触部位(称为线粒体相关膜(MAMs))在与营养不良、肥胖和营养不良双重负担(DBM)相关的胰岛素分泌功能障碍中的作用。方法:将大鼠胰腺INS-1E β细胞在不含氨基酸的培养基中,添加1 × (对照)或0.25 × (氨基酸限制)的氨基酸溶液培养48 h,然后将细胞暴露于脂肪酸混合物中48 h。雄性C57BL/6小鼠喂食正常蛋白饮食(14 %蛋白质)或蛋白质限制饮食(6 %蛋白质)6 周,随后喂食高脂肪饮食(35 % kcal) 12 周。通过原位接近结扎试验和透射电镜评估er -线粒体相互作用。结果:我们的研究结果表明,蛋白质限制减少了胰腺β细胞中er -线粒体的接触,导致线粒体代谢和葡萄糖刺激胰岛素分泌(GSIS)下降。相比之下,肥胖增加了胰β细胞的er -线粒体接触点、线粒体代谢和GSIS,而不改变生存能力。DBM导致er -线粒体接触显著增加,线粒体钙水平升高,活性氧产生增加,细胞死亡,共同导致肥胖背景下GSIS反应受损。结论/解释:这些数据表明MAMs在营养失调(如营养不良、肥胖和DBM)期间的GSIS中起着至关重要的作用。重要的是,MAMs的变化先于GSIS损伤,因此靶向这些相互作用可能会防止β细胞功能的进一步破坏。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Metabolism: clinical and experimental
医学-内分泌学与代谢
CiteScore
18.90
自引率
3.10%
发文量
310
审稿时长
16 days
期刊介绍:
Metabolism upholds research excellence by disseminating high-quality original research, reviews, editorials, and commentaries covering all facets of human metabolism.
Consideration for publication in Metabolism extends to studies in humans, animal, and cellular models, with a particular emphasis on work demonstrating strong translational potential.
The journal addresses a range of topics, including:
- Energy Expenditure and Obesity
- Metabolic Syndrome, Prediabetes, and Diabetes
- Nutrition, Exercise, and the Environment
- Genetics and Genomics, Proteomics, and Metabolomics
- Carbohydrate, Lipid, and Protein Metabolism
- Endocrinology and Hypertension
- Mineral and Bone Metabolism
- Cardiovascular Diseases and Malignancies
- Inflammation in metabolism and immunometabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


